In a nutshell
Vortioxetine combines serotonin reuptake inhibition with direct modulation of multiple serotonin receptors, potentially offering advantages for patients with cognitive symptoms of depression. Its main clinical advantage appears to be a favorable tolerability profile, particularly regarding sexual dysfunction and discontinuation symptoms.

- Choosing vortioxetine over other antidepressants:
- Preferred for patients with prominent cognitive symptoms of depression
- Lower risk of sexual dysfunction compared to SSRIs
- Minimal discontinuation symptoms due to long half-life
- Low risk of drug interactions (except with CYP2D6 inhibitors/inducers)
- Consider alternatives when:
- Patients sensitive to GI side effects (high rates of nausea)
- Treating anxiety disorders (insufficient evidence for GAD)
- Cost is a concern (newer branded medication)
- Concurrent use of strong CYP2D6 inhibitors/inducers
Pharmacodynamics and mechanism of action
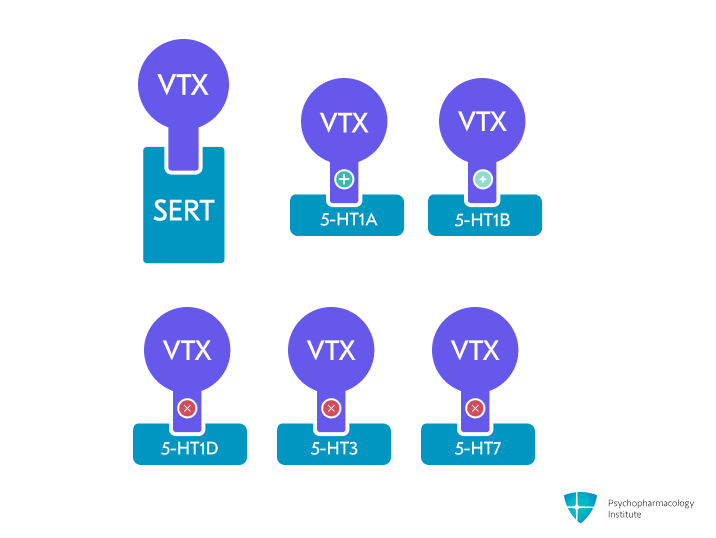
- Multimodal mechanism combining serotonin reuptake inhibition with direct modulation of serotonin receptors [1]
- Primary mechanism: Potent serotonin reuptake inhibition through SERT blockade [2]
- Direct receptor activity [2,3]
- Agonist: 5-HT1A receptor
- Partial agonist: 5-HT1B receptor
- Antagonist: 5-HT1D, 5-HT3, 5-HT7 receptors
- Preclinical findings and potential implications
- While vortioxetine’s receptor binding profile distinguishes it from traditional SSRIs, the clinical significance of these pharmacological differences continues to be investigated [2].
- 5-HT1A agonism
- May accelerate antidepressant response through somatodendritic receptor desensitization [2,4]
- 5-HT3 antagonism
- May enhance anxiolytic and antidepressant effects [2]
- 5-HT7 antagonism
- Potentially enhances antidepressant effects when combined with SERT inhibition [2]
- May be linked to pro-cognitive effects [5]
- Combined 5-HT1B partial agonism and SERT inhibition
- Increased serotonin levels in the prefrontal cortex and enhanced serotonergic neuron firing have been described in animal models [6].
Pharmacokinetics
Metabolism
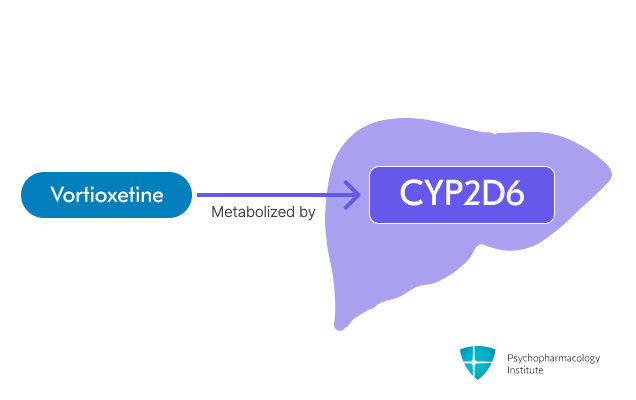
- Primarily metabolized through oxidation via CYP2D6 [3]
- Minor pathways involve other CYP450 isoenzymes (CYP3A4/5, CYP2C19, CYP2C9, etc).
-
CYP2D6 metabolism
- CYP2D6 metabolizer status affects plasma concentrations
- Poor metabolizers exhibit approximately double the plasma concentrations compared to extensive metabolizers.
- Maximum recommended dose for CYP2D6 poor metabolizers is 10 mg/day [3]
-
Vortioxetine levels increased by
- Strong CYP2D6 inhibitors (bupropion, fluoxetine, paroxetine, quinidine
- Reduce vortioxetine dose by 50%
- Return to original dose when inhibitor is discontinued
- Strong CYP2D6 inhibitors (bupropion, fluoxetine, paroxetine, quinidine
-
Vortioxetine levels reduced by
- Strong CYP inducers (rifampin, carbamazepine, phenytoin)
- Consider increasing vortioxetine dose
- Maximum dose should not exceed 3 times the original dose
- Return to original dose within 14 days of discontinuing the inducer [7]
- Strong CYP inducers (rifampin, carbamazepine, phenytoin)
-
Serotonergic agents
- Monitor for serotonin syndrome with other serotonergic medications (SSRIs, SNRIs, triptans, tricyclic antidepressants).
- Also applies to tramadol, tryptophan, and St. John’s Wort
-
Contraindicated with MAOIs [3]
- Due to vortioxetine’s long half-life, a 21-day washout period is required after discontinuation before starting an MAOI.
- A 14-day washout period is required after discontinuing an MAOI before initiating vortioxetine.
Half-life
- Vortioxetine half-life is approximately 66 hours.
False-positive drug tests
- Vortioxetine can trigger false-positive methadone results in urine immunoassays [3]
- Confirmatory testing via chromatographic methods is recommended for positive screens.
Dosage forms
Dosage forms
- Immediate-release:
- Film-coated tablets
- 5 mg, 10 mg, 20 mg
- Trintellix
- Film-coated tablets
- Formulation considerations
- Tablets can be taken with or without food, at either morning or bedtime.
Indications
FDA-Approved Indications
Major depressive disorder
- First-line antidepressant treatment option for MDD based on efficacy and favorable tolerability profile [8,9]
- Shows advantages over other antidepressants when considering both efficacy and acceptability in network meta-analyses [10]
- Cognitive effects:
- The labeling includes data showing improvements in cognitive processing speed (as measured by the Digit Symbol Substitution Test)
- Despite seeking separate regulatory approval, vortioxetine was not approved for cognitive symptoms of depression as a standalone indication.
- May be particularly beneficial for patients with cognitive symptoms (problems with concentration, memory, and executive functioning), and age-related cognitive decline [11,12]
- Recent systematic review reports superior efficacy compared to SSRIs [13]
- Half to two-thirds of the observed effect of vortioxetine on cognitive improvement is a direct effect on cognition itself, rather than being a consequence of improvements in depressive symptoms.
- Dosing:
- Starting dose: 10 mg once daily
- Sensitive patients: Start at 5 mg once daily for patients who experience nausea or who do not tolerate higher doses
- Target dose: 20 mg/day
- Maximum dose:
- General population: 20 mg/day
- CYP2D6 poor metabolizers: 10 mg/day [3]
- May be taken day or night, independent of food intake
- Starting dose: 10 mg once daily
Off-label Uses
Generalized anxiety disorder
- The evidence does not support vortioxetine for use in GAD [14,15]
Obsessive compulsive disorder
- There are no double-blind studies investigating vortioxetine’s efficacy in OCD [16]
- However, case report indicates vortioxetine’s potential role for depressed patients with obsessive symptoms [17], or in OCD patients unsuitable for TCAs or unresponsive to SSRIs [18,19].
Side effects
Most common side effects
Gastrointestinal
- Nausea (21-32% incidence)
- Most common adverse effect leading to discontinuation
- Usually occurs in the first week, with 15-20% experiencing nausea after 1-2 days of treatment [3].
- Dose-related: Approximately 10% still experience nausea at treatment end with doses 10-20mg/day
- Can be minimized by taking it with food
- Diarrhea (7-10% incidence)
- Dry mouth (7% incidence)
- Constipation (3-6% incidence)
- Vomiting (3-6% incidence)
Other common side effects
- Discontinuation syndrome
- Lower risk compared to other antidepressants due to vortioxetine’s 66-hour half-life [20,21]
- Clinical trials and observational data suggest low incidence and/or similar to placebo [21,22]
- For 15-20mg/day doses, a reduction to 10mg/day for one week before discontinuation is recommended [3]
- Antidepressant-induced sexual dysfunction
- Lower rates of sexual dysfunction compared to other antidepressants [21,23]
- However, spontaneous reporting likely underestimates actual prevalence [24]
- Even though the effect is dose-dependent, treatment-emergent sexual dysfunction rates remain comparable to placebo [25]
- May improve sexual function in patients switching from high-risk antidepressants (e.g., citalopram, paroxetine, or sertraline) [23]
- Consider lower doses for patients concerned about sexual side effects
- Use structured assessment tools like the ASEX rather than relying on spontaneous reporting
- Monitor for dose-dependent effects
- Lower rates of sexual dysfunction compared to other antidepressants [21,23]
- Dizziness (6-9% incidence)
- Abnormal dreams (2-3% incidence)
- Pruritus (2-3% incidence)
Severe side effects
- Serotonin syndrome
- Risk increases with other serotonergic drugs
- Has also been reported in monotherapy [26]
- Use caution when combining vortioxetine with serotonergic medications, especially during treatment initiation or dose adjustments [3].
- Bleeding risk
- Serotonergic antidepressants (SSRIs, SNRIs) may increase bleeding risk, particularly with concurrent NSAIDs, antiplatelets or anticoagulants.
- Vortioxetine may share this risk, though current evidence is limited
- In healthy volunteers, coadministration with warfarin or aspirin did not alter coagulation parameters [27].
- Clinical data in depressed populations remain sparse; monitor closely when combined with anticoagulants, especially at initiation [28].
- Hyponatremia
- Antidepressant therapy can precipitate SIADH and clinically significant hyponatremia.
- Ranking of risk [29]:
- MAOIs > SNRIs > SSRIs > TCAs > Mirtazapine
- Vortioxetine was not included in this meta-analysis, but cases have been documented [30–32].
- Special caution in elderly patients, patients taking diuretics or who are otherwise volume-depleted
Use in special populations
Pregnancy
- First-trimester safety
- Animal reproductive studies of vortioxetine show no evidence of increased congenital malformation risk during pregnancy.
- Limited human data from a case series of 17 first-trimester exposures reported [33]
- Pregnancy complications
- Late pregnancy exposure may be associated with [3]
- Neonatal adaptation syndrome
- Persistent pulmonary hypertension of the newborn (PPHN)
- Similar to other serotonergic antidepressants
- Late pregnancy exposure may be associated with [3]
Breastfeeding
- Vortioxetine is present in breast milk.
- FDA prescribing information notes lack of data on effects on breastfed infants and milk production [3]
- Available case reports suggest minimal infant exposure, but few cases and limited follow-up data [34]
- Relative infant dose: 1.1-1.7% of maternal dose
- Based on data from only 3 women taking 10-20 mg/day
- One additional case report describes successful breastfeeding for one year while on medication [33]
- No follow-up information provided on infant outcomes
- Breastfed infants should be monitored for
- Sedation
- Poor feeding patterns
- Weight gain adequacy
- Development
Hepatic impairment
- No dose adjustment needed
Renal impairment
- No dose adjustment needed
Elderly
- No dose adjustment recommended [3]
- Starting dose 5 mg/day recommended [35]
- Caution with doses >10 mg/day due to
- Increased risk of hyponatremia
- Higher exposure (up to 27%) compared to younger adults
Obesity
- Mean elimination half-life prolonged by 48% in patients with BMI ≥35 kg/m² compared to normal BMI
- Consider extended washout period when switching to MAOIs in patients with obesity [36]
Brand names
- US: Trintellix
- Canada: Trintellix
- Other countries/regions: Acsodix, Brintellix, Brivor, Depratiox, Fonksera, Kelac, Lupivor, Procetina, Torvox, Trivoxetin, Trintogen, Valquir, Vantaxa, Vectax, Vipca, Vormind, Vorpix, Vorsero, Vortica, Vortidif, Vortiox, Vortiray, Vorxetil, Voxigain, Voxitin, Vorellix, Vivirum, Vorti, Vorasan, Vurtuoso, Xomet
References
- Köhler, S., Cierpinsky, K., Kronenberg, G., & Adli, M. (2016). The serotonergic system in the neurobiology of depression: Relevance for novel antidepressants. Journal of Psychopharmacology, 30(1), 13–22. https://doi.org/10.1177/0269881115609072
- Sowa-Kućma, M., Pańczyszyn-Trzewik, P., Misztak, P., Jaeschke, R. R., Sendek, K., Styczeń, K., Datka, W., & Koperny, M. (2017). Vortioxetine: A review of the pharmacology and clinical profile of the novel antidepressant. Pharmacological Reports, 69(4), 595–601. https://doi.org/10.1016/j.pharep.2017.01.030
- Trintellix FDA.pdf. (n.d.).
- Celada, P., Bortolozzi, A., & Artigas, F. (2013). Serotonin 5-HT1A Receptors as Targets for Agents to Treat Psychiatric Disorders: Rationale and Current Status of Research. CNS Drugs, 27(9), 703–716. https://doi.org/10.1007/s40263-013-0071-0
- Sanchez, C., Asin, K. E., & Artigas, F. (2015). Vortioxetine, a novel antidepressant with multimodal activity: Review of preclinical and clinical data. Pharmacology & Therapeutics, 145, 43–57. https://doi.org/10.1016/j.pharmthera.2014.07.001
- Pehrson, A. L., Cremers, T., Bétry, C., van der Hart, M. G. C., Jørgensen, L., Madsen, M., Haddjeri, N., Ebert, B., & Sanchez, C. (2013). Lu AA21004, a novel multimodal antidepressant, produces regionally selective increases of multiple neurotransmitters–a rat microdialysis and electrophysiology study. European Neuropsychopharmacology: The Journal of the European College of Neuropsychopharmacology, 23(2), 133–145. https://doi.org/10.1016/j.euroneuro.2012.04.006
- Chen, G., Højer, A.-M., Areberg, J., & Nomikos, G. (2018). Vortioxetine: Clinical Pharmacokinetics and Drug Interactions. Clinical Pharmacokinetics, 57(6), 673–686. https://doi.org/10.1007/s40262-017-0612-7
- Lam, R. W., Kennedy, S. H., Adams, C., Bahji, A., Beaulieu, S., Bhat, V., Blier, P., Blumberger, D. M., Brietzke, E., Chakrabarty, T., Do, A., Frey, B. N., Giacobbe, P., Gratzer, D., Grigoriadis, S., Habert, J., Ishrat Husain, M., Ismail, Z., McGirr, A., … Milev, R. V. (2024). Canadian Network for Mood and Anxiety Treatments (CANMAT) 2023 Update on Clinical Guidelines for Management of Major Depressive Disorder in Adults: Réseau canadien pour les traitements de l’humeur et de l’anxiété (CANMAT) 2023 : Mise à jour des lignes directrices cliniques pour la prise en charge du trouble dépressif majeur chez les adultes. Can. J. Psychiatry, 69(9), 641–687. https://doi.org/10.1177/07067437241245384
- Qaseem, A., Owens, D. K., Etxeandia-Ikobaltzeta, I., Tufte, J., Cross, J. T., Wilt, T. J., & Clinical Guidelines Committee of the American College of Physicians. (2023). Nonpharmacologic and Pharmacologic Treatments of Adults in the Acute Phase of Major Depressive Disorder: A Living Clinical Guideline From the American College of Physicians. Annals of Internal Medicine, 176(2), 239–252. https://doi.org/10.7326/M22-2056
- Cipriani, A., Furukawa, T. A., Salanti, G., Chaimani, A., Atkinson, L. Z., Ogawa, Y., Leucht, S., Ruhe, H. G., Turner, E. H., Higgins, J. P. T., Egger, M., Takeshima, N., Hayasaka, Y., Imai, H., Shinohara, K., Tajika, A., Ioannidis, J. P. A., & Geddes, J. R. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet, 391(10128), 1357–1366. https://doi.org/10.1016/s0140-6736(17)32802-7
- Lenze, E. J., Stevens, A., Waring, J. D., Pham, V. T., Haddad, R., Shimony, J., Miller, J. P., & Bowie, C. R. (2020). Augmenting Computerized Cognitive Training With Vortioxetine for Age-Related Cognitive Decline: A Randomized Controlled Trial. American Journal of Psychiatry, 177(6), 548–555. https://doi.org/10.1176/appi.ajp.2019.19050561
- Huang, I.-C., Chang, T.-S., Chen, C., & Sung, J.-Y. (2022). Effect of Vortioxetine on Cognitive Impairment in Patients With Major Depressive Disorder: A Systematic Review and Meta-analysis of Randomized Controlled Trials. International Journal of Neuropsychopharmacology, 25(12), 969–978. https://doi.org/10.1093/ijnp/pyac054
- Blumberg, M. J., Vaccarino, S. R., & McInerney, S. J. (2020). Procognitive Effects of Antidepressants and Other Therapeutic Agents in Major Depressive Disorder: A Systematic Review. The Journal of Clinical Psychiatry, 81(4). https://doi.org/10.4088/JCP.19r13200
- Bandelow, B., Allgulander, C., Baldwin, D. S., Costa, D. L. da C., Denys, D., Dilbaz, N., Domschke, K., Eriksson, E., Fineberg, N. A., Hättenschwiler, J., Hollander, E., Kaiya, H., Karavaeva, T., Kasper, S., Katzman, M., Kim, Y.-K., Inoue, T., Lim, L., Masdrakis, V., … Zohar, J. (2023). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders – Version 3. Part I: Anxiety disorders. World J. Biol. Psychiatry, 24(2), 79–117. https://doi.org/10.1080/15622975.2022.2086295
- Qin, B., Huang, G., Yang, Q., Zhao, M., Chen, H., Gao, W., & Yang, M. (2019). Vortioxetine treatment for generalised anxiety disorder: A meta-analysis of anxiety, quality of life and safety outcomes. BMJ Open, 9(11), e033161. https://doi.org/10.1136/bmjopen-2019-033161
- Pizarro, M., Fontenelle, L. F., Paravidino, D. C., Yücel, M., Miguel, E. C., & De Menezes, G. B. (2014). An updated review of antidepressants with marked serotonergic effects in obsessive–compulsive disorder. Expert Opinion on Pharmacotherapy, 15(10), 1391–1401. https://doi.org/10.1517/14656566.2014.914493
- Santayana, G. P. de, Landera, R., Juncal, M., Porta, O., Sánchez, L., Gómez, M., Núñez, N., & Pérez, M. (2017). Vortioxetine Efficiency in Controlling Obsessive Symptoms in Patients with Depression. A Case Report. European Psychiatry, 41(S1), S715–S715. https://doi.org/10.1016/j.eurpsy.2017.01.1283
- Jiménez-Fernández, B., Motta-Rojas, N. V., Iborra-Vicheto, X., & Cuevas-Esteban, J. (2024). Use of vortioxetine in treating obsessive-compulsive disorder: A case report. European Psychiatry, 67(S1), S632–S632. https://doi.org/10.1192/j.eurpsy.2024.1309
- De Berardis, D., Olivieri, L., Nappi, F., Rapini, G., Vellante, F., Matarazzo, I., Serroni, N., & Di Giannantonio, M. (2017). Vortioxetine and Aripiprazole Combination in Treatment-Resistant Obsessive-Compulsive Disorder: A Case Report. Journal of Clinical Psychopharmacology, 37(6), 732. https://doi.org/10.1097/JCP.0000000000000801
- Areberg, J., Petersen, K. B., Chen, G., & Naik, H. (2014). Population Pharmacokinetic Meta-Analysis of Vortioxetine in Healthy Individuals. Basic & Clinical Pharmacology & Toxicology, 115(6), 552–559. https://doi.org/10.1111/bcpt.12256
- Baldwin, D. S., Chrones, L., Florea, I., Nielsen, R., Nomikos, G. G., Palo, W., & Reines, E. (2016). The safety and tolerability of vortioxetine: Analysis of data from randomized placebo-controlled trials and open-label extension studies. Journal of Psychopharmacology (Oxford, England), 30(3), 242–252. https://doi.org/10.1177/0269881116628440
- Siwek, M., Chrobak, A. A., Gorostowicz, A., Krupa, A. J., & Dudek, D. (2021). Withdrawal Symptoms Following Discontinuation of Vortioxetine—Retrospective Chart Review. Pharmaceuticals, 14(5), 451. https://doi.org/10.3390/ph14050451
- Jacobsen, P. L., Mahableshwarkar, A. R., Chen, Y., Chrones, L., & Clayton, A. H. (2015). Effect of Vortioxetine vs. Escitalopram on Sexual Functioning in Adults with Well-Treated Major Depressive Disorder Experiencing SSRI-Induced Sexual Dysfunction. The Journal of Sexual Medicine, 12(10), 2036–2048. https://doi.org/10.1111/jsm.12980
- de Boer, M. K., & Schoevers, R. A. (2017). Methodological differences as an explanation for the divergent results of studies on sexual dysfunction related to the use of vortioxetine. Journal of Psychopharmacology, 31(3), 389–390. https://doi.org/10.1177/0269881116681520
- Jacobsen, P. L., Mahableshwarkar, A. R., Palo, W. A., Chen, Y., Dragheim, M., & Clayton, A. H. (2016). Treatment-emergent sexual dysfunction in randomized trials of vortioxetine for major depressive disorder or generalized anxiety disorder: A pooled analysis. CNS Spectrums, 21(5), 367–378. https://doi.org/10.1017/S1092852915000553
- Ong, C. Y., & Vasanwala, F. F. (2018). Diaphoresis: A Presentation of Serotonin Syndrome From Vortioxetine. The Primary Care Companion for CNS Disorders, 20(3), 26849. https://doi.org/10.4088/PCC.17l02191
- Chen, G., Zhang, W., & Serenko, M. (2015). Lack of effect of multiple doses of vortioxetine on the pharmacokinetics and pharmacodynamics of aspirin and warfarin. The Journal of Clinical Pharmacology, 55(6), 671–679. https://doi.org/10.1002/jcph.456
- Rahman, A. A., Platt, R. W., Beradid, S., Boivin, J.-F., Rej, S., & Renoux, C. (2024). Concomitant Use of Selective Serotonin Reuptake Inhibitors With Oral Anticoagulants and Risk of Major Bleeding. JAMA Network Open, 7(3), e243208. https://doi.org/10.1001/jamanetworkopen.2024.3208
- Gheysens, T., Van Den Eede, F., & De Picker, L. (2024). The risk of antidepressant-induced hyponatremia: A meta-analysis of antidepressant classes and compounds. European Psychiatry, 67(1), e20. https://doi.org/10.1192/j.eurpsy.2024.11
- D’Agostino, A., English, C. D., & Rey, J. A. (2015). Vortioxetine (Brintellix): A New Serotonergic Antidepressant. Pharmacy and Therapeutics, 40(1), 36–40. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4296590/
- Pelayo-Terán, J. M., Martínez-Pérez, M. M., & Zapico-Merayo, Y. (2017). Safety in the use of antidepressants: Vortioxetine-induce hyponatremia in a case report. Revista De Psiquiatria Y Salud Mental, 10(4), 219–220. https://doi.org/10.1016/j.rpsm.2017.07.001
- Sasaki, T., Shindo, Y., Kikuchi, K., Kawamata, Y., Sugawara, N., & Yasui‐Furukori, N. (2024). Vortioxetine‐induced syndrome of inappropriate secretion of antidiuretic hormone: A case report. Neuropsychopharmacology Reports, 44(2), 479–481. https://doi.org/10.1002/npr2.12438
- Shweiki, S., & Diav-Citrin, O. (2021). Pregnancy outcome after first trimester exposure to vortioxetine: A case series. Birth Defects Research, 113(6), 511–515. https://doi.org/10.1002/bdr2.1864
- Marshall, K., Datta, P., Rewers-Felkins, K., Krutsch, K., Baker, T., & Hale, T. W. (2021). Transfer of the Serotonin Modulator Vortioxetine into Human Milk: A Case Series. Breastfeeding Medicine: The Official Journal of the Academy of Breastfeeding Medicine, 16(10), 843–845. https://doi.org/10.1089/bfm.2021.0074
- TrintellixCanada.PDF. (n.d.).
- Greenblatt, D. J., Harmatz, J. S., & Chow, C. R. (2018). Vortioxetine Disposition in Obesity: Potential Implications for Patient Safety. Journal of Clinical Psychopharmacology, 38(3), 172–179. https://doi.org/10.1097/JCP.0000000000000861



