Venlafaxine (Effexor) is an SNRI that is metabolized to O-desmethylvenlafaxine or desvenlafaxine. In 2008 this active metabolite was approved as an antidepressant (Pristiq). In this multimedia tutorial we discuss what the two drugs have in common and their differences, we also explore mechanisms of action, indications, pharmacokinetics, adverse effects and dosing guidelines.
Summary points:
- They are similar in terms of efficacy, pharmacodynamics and side effects profile.
- There are differences in pharmacokinetic aspects and dosing guidelines.
Mechanism of Action and Pharmacodynamics
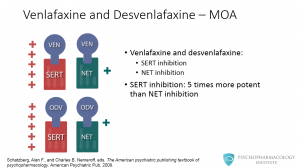
Both venlafaxine and o- desvenlafaxine inhibit the neuronal reuptake of serotonin. They also inhibit the norepinephrine transporter, but according to in vitro studies, the affinity of both drugs is significantly lower for the norepinephrine transporter compared to the SERT. Venlafaxine is approximately 5 times more potent in vitro as SERT inhibitor versus norepinephrine reuptake inhibitor. This higher serotonergic affinity has been linked to venlafaxine’s side effects profile.
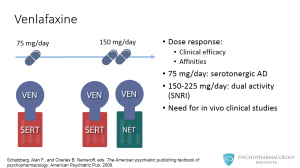
There is evidence from experimental and clinical data suggesting that there is a dose-response relationship in terms of clinical efficacy and pharmacological affinities. At its lower therapeutic dose (75 mg/day), venlafaxine blocks the reuptake only of serotonin. So it can be said that at 75 mg venlafaxine works as an SSRI. As the dose increases, so does its noradrenergic effect. It is believed that at a range of 150-225 mg a day, venlafaxine shows its dual activity as both serotonin and norepinephrine reuptake blocker. There is one caveat though, this hypothesis cannot be fully tested until a radioactive ligand is developed, so that in vivo clinical studies can be performed. An unrelated example of this type of studies are PET studies that measure D2 receptor occupancy and correlate it with antipsychotic activity.
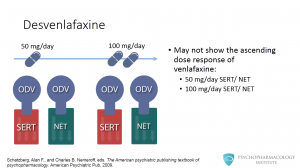
Desvenlafaxine may not show the ascending dose response of venlafaxine. At 50 mg/day the drug inhibits both serotonin and norepinephrine reuptake, affinity doesn’t appear to change when the dose is increased to 100 mg/day.
Indications
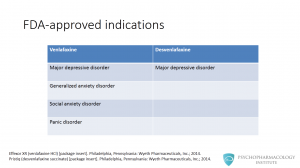
What are the indications for venlafaxine and desvenlafaxine? Venlafaxine is FDA-approved for major depressive disorder and anxiety disorders, these include: generalized anxiety disorder, social anxiety disorder and panic disorder. Desvenlafaxine is only approved for major depressive disorder.
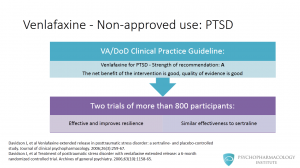
Are there non approved uses with a good level of evidence for venlafaxine? Venlafaxine has also been studied for the treatment of posttraumatic stress disorder. The US VA/Department of Defense clinical practice guidelines recommend venlafaxine as a first line agent for PTSD. The strength of this recommendation is A, this means that the net benefit of the intervention is good and the quality of the evidence is also good. The guidelines reference two trials of more than 800 participants with non-combat related PTSD. One describes that it is effective and improves resilience and the other suggests that venlafaxine has similar effectiveness to sertraline.
Pharmacokinetics
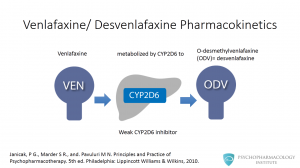
Venlafaxine undergoes metabolism in the liver by the cytochrome P450 2D6 to O-desmethylvenlafaxine, the active metabolite that is commercially available as aan ntidepressant. Venlafaxine is also a weak inhibitor of CYP2D6, but there are no clinically relevant interactions with most coadministered medications. MAOIs are an exception to this.
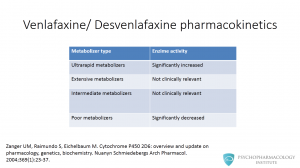
Let’s pause for a minute to discuss a brief concept relevant to venlafaxine pharmacokinetics. There are several metabolizer types, as you can see in this table, ultrarapid, extensive, intermediate and poor metabolizers. Only poor metabolizers are relevant to our discussion here. Poor metabolizers have significantly decreased CYP450 2D6 activity.
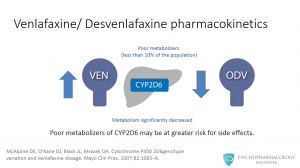
This means that in the case of venlafaxine, this population might have increased concentrations of the parent drug relative to o-desvenlafaxine. Given that the parent drug and o-desvenlafaxine are nearly pharmacologically equipotent, poor metabolizers of CYP2D6 may be at greater risk for side effects. This group of patients could potentially be better candidates for therapy with desvenlafaxine than the parent drug.
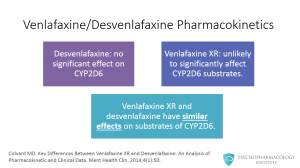
Since desvenlafaxine has no significant effect on CYP2D6 at therapeutic doses, it is promoted as a drug with lower potential for drug-drug interactions with CYP2D6 substrates. What is interesting here is that venlafaxine is a weak inhibitor of CYP2D6, and the extended release formulation is unlikely to significantly affect CYP2D6 substrates. This means that venlafaxine XR and desfenlafaxine have similar effects on substrates of CYP2D6 at recommended doses.
Adverse Effects – Tolerability
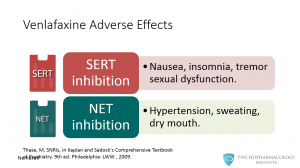
Venlafaxine has a tolerability profile linked to increased serotonergic and noradrenergic inhibition. Side effects associated with serotonin reuptake inhibition include: nausea, insomnia, tremor and sexual dysfunction. Some effects associated with norepinephrine reuptake inhibition include hypertension, sweating and dry mouth. In addition to allowing a once-daily dosing, one advantage of the XR formulation is a somewhat lower incidence of nausea during the first weeks of therapy.

Venlafaxine is one of the the antidepressants most commonly associated with discontinuation syndrome. In addition to allowing a once-daily dosing, one advantage of the XR formulation is a somewhat lower incidence of nausea during the first weeks of therapy.
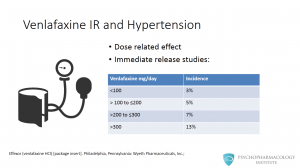
Hypertension is an important side effect to bear in mind when prescribing venlafaxine. Higher dose therapy is associated with an increased risk of sustained elevations of blood pressure, hypertension is a dose related side effect. This table shows data from the IR release studies. It shows that for a dose lower 100mg/day the incidence is of 3%, when dosing around 200 to 300 mg/day, the incidence is of 7%. Above 300mg/day the number goes to 13%.
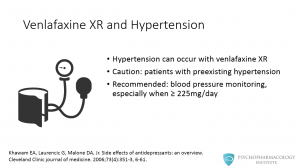
What about the XR formulation? Hypertension can also occur with venlafaxine XR, especially at higher doses. Prescribers should be cautious when using this medication for patients with preexisting hypertension. The manufacturer recommends blood pressure to be monitored regularly, especially when using venlafaxine XR at doses of 225 mg or more per day.
Dosing

The usual dosage range recommended by the manufacturer is between 75 to 225 mg/day. Venlafaxine can be dosed higher by experienced prescribers, but always keeping in mind the possibility of drug-induced hypertension. The extended release formulation allows once daily dosing, while the immediate release formulation needs to be given in two to three doses a day.
The dosage forms include:
– Capsules (XR): 37.5 mg, 75 mg, 150 mg
– Tablets: 37.5 mg, 75 mg, 150 mg, 225 mg
– Scored tablets: 25 mg, 37.5 mg 75 mg, 100 mg

For most patients, the recommended starting dose for venlafaxine XR is 75 mg/day, administered in a single dose. For some patients, it may be desirable to start at 37.5 mg/day for 4 to 7 days, to allow new patients to adjust to the medication before increasing to 75 mg/day. Patients not responding to the initial 75 mg/day dose may benefit from dose increases to a maximum of approximately 225 mg/day. The manufacturer also makes clear that while the maximum recommended dose for moderately depressed outpatients is also 225 mg/day, more severely depressed inpatients in one study of the development program responded to a mean dose of 350 mg/day.

Desvenlafaxine dose is of 50 mg/day. Dosage forms include extended release tablets of 50 and 100 mg. This is an important difference with venlafaxine, the development trials showed no evidence that doses greater than 50 mg/day confer any additional benefits In clinical studies, doses of 50 mg to 400 mg per day were shown to be effective, although no additional benefit was demonstrated at doses greater than 50 mg per day and adverse reactions and discontinuations were more frequent at higher doses.
— Note: this tutorial is also available in spanish: Venlafaxina y desvenlafaxina: similitudes y diferencias
References and Further Reading
- Schatzberg, Alan F., and Charles B. Nemeroff, eds. The American psychiatric publishing textbook of psychopharmacology. American Psychiatric Pub, 2009.
- Effexor XR (venlafaxine HCl) [package insert]. Philadelphia, Pennsylvania: Wyeth Pharmaceuticals, Inc.; 2014.
- Pristiq (desvenlafaxine succinate) [package insert]. Philadelphia, Pennsylvania: Wyeth Pharmaceuticals, Inc.; 2014
- Davidson J, et al Venlafaxine extended release in posttraumatic stress disorder: a sertraline- and placebo-controlled study . Journal of clinical psychopharmacology. 2006;26(3):259-67.
- Davidson J, et al Treatment of posttraumatic stress disorder with venlafaxine extended release: a 6-month randomized controlled trial. Archives of general psychiatry. 2006;63(10):1158-65.
- Janicak, P G., Marder S R., and. Pavuluri M N. Principles and Practice of Psychopharmacotherapy. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2010.
- Zanger UM, Raimundo S, Eichelbaum M. Cytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistry. Nuanyn Schmiedebergs Arch Pharmacol. 2004;369(1):23-37.
- McAlpine DE, O’Kane DJ, Black JL, Mrazek DA. Cytochrome P450 2D6genotype variation and venlafaxine dosage. Mayo Clin Proc. 2007;82:1065–8.
- Colvard MD. Key Differences Between Venlafaxine XR and Desvenlafaxine: An Analysis of Pharmacokinetic and Clinical Data. Ment Health Clin. 2014;4(1):50.
- Thase, M. SNRIs, in Kaplan and Sadock’s Comprehensive Textbook of Psychiatry. 9th ed. Philadelphia: LWW , 2009.
- Khawam EA, Laurencic G, Malone DA, Jr. Side effects of antidepressants: an overview. Cleveland Clinic journal of medicine. 2006;73(4):351-3, 6-61.
- Stahl, S M. The Prescriber’s Guide. 4th ed. New York: Cambrigde University Press; 2011



