Risperidone is a second-generation antipsychotic metabolized by the enzyme cytochrome P450 2D6 (CYP2D6). It is available as long-acting injection (depot injection) as well as oral formulations. This clinical summary highlights pharmacokinetic features relevant to mental health prescribers.
Formulations
- Oral formulations:
- Tablets
- Orally disintegrating tablets
- Liquid formulation
- Long-acting injection: risperidone microspheres (Risperdal Consta)
General pharmacokinetics
Distribution
- Volume of distribution: 1-2 L/kg.
- Risperidone binds to albumin and alpha1-acid glycoprotein.
- Plasma protein binding:
- For risperidone: 90%
- For 9-hydroxyrisperidone: 77%
Metabolism
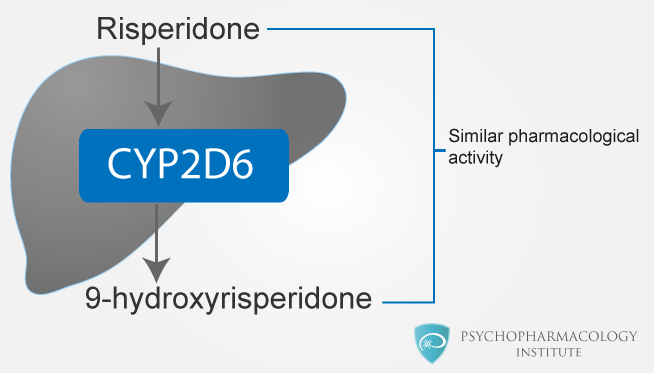
Risperidone metabolism by CYP2D6
- CYP 2D6 is the enzyme that catalyzes hydroxylation of risperidone to 9-hydroxyrisperidone.
- The metabolite 9-hydroxyrisperidone has similar pharmacological activity as risperidone.
- CYP 2D6 is subject to genetic polymorphism:
- Extensive metabolizers convert risperidone rapidly into 9-hydroxyrisperidone
- Poor CYP 2D6 metabolizers convert risperidone much more slowly.
- Pharmacokinetics of risperidone and 9-hydroxyrisperidone combined after single and multiple doses are similar in extensive and poor metabolizers.
Pharmacokinetics of Oral Formulations
Absorption
- Orally disintegrating tablets and oral solution are bioequivalent to tablets.
- Rapidly absorbed after oral administration.
- Peak plasma levels achieved within 1 hour.
- Linear pharmacokinetics.
- Time to reach steady state ( between 4 and 5 half-lives for all drugs):
- For risperidone:
- 1 day in extensive metabolizers.
- 5 days in poor metabolizers.
- For 9-hydroxyrisperidone:
- 5-6 days, measured in extensive metabolizers.
- For risperidone:
- Food effect: risperidone can be administered with or without meals.
Excretion
- Apparent half-life of risperidone:
- 3 hours in extensive metabolizers
- 20 hours in poor metabolizers
- Apparent half-life of 9-hydroxyrisperidone:
- 21 hours in extensive metabolizers
- 30 hours in poor metabolizers
Pharmacokinetics of Long-Acting Injection (Risperidone Microspheres)
- Risperidone microspheres release considerable amounts of drug 3 weeks after injection.
- Long-acting risperidone should be supplemented with oral risperidone for 3 weeks.
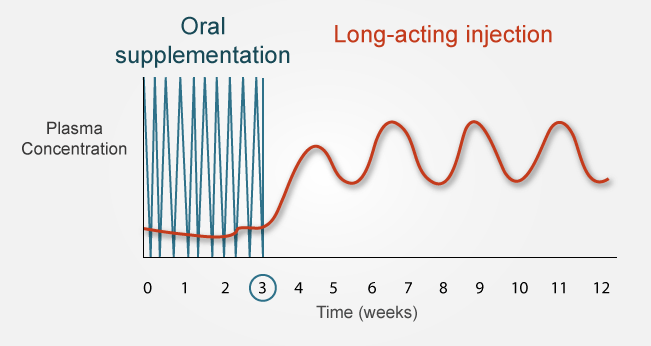
- Apparent half-life or risperidone plus 9-hydroxyrisperidone is 3 to 6 days (related to the erosion of the microspheres and subsequent absorption of risperidone).
Related Information on Risperidone
References
- Janicak, P G., Marder S R., and. Pavuluri M N. Principles and Practice of Psychopharmacotherapy. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2010.
- Stahl, S M. The Prescriber’s Guide. 4th ed. New York: Cambrigde University Press; 2011
- Janssen Pharmaceuticals, Inc. Risperdal prescribing information. Retrieved from http://www.janssenpharmaceuticalsinc.com/assets/risperdal.pdf. [retrieval date: April 12, 2013]
- Janssen Pharmaceuticals, Inc. Risperdal Consta prescribing information. Retrieved from http://www.janssencns.com/risperdal-prescribing-information



