In a nutshell
- Fluvoxamine (Luvox) is approved for OCD in the U.S. for pediatric and adult use.
- There is no clear evidence that fluvoxamine is more effective than other SSRIs for OCD.
- It is approved for depression in the UK.
- A strong inhibitor of CYP1A2. Should not be used with MAOIs.
Pharmacodynamics
- Animal studies reveal that:
- Potential clinical implications of sigma-1 receptor agonism:3
- Psychotic depression: It has been suggested that sigma-1 agonism might be involved in fluvoxamine’s effects on symptoms of psychotic depression. 4
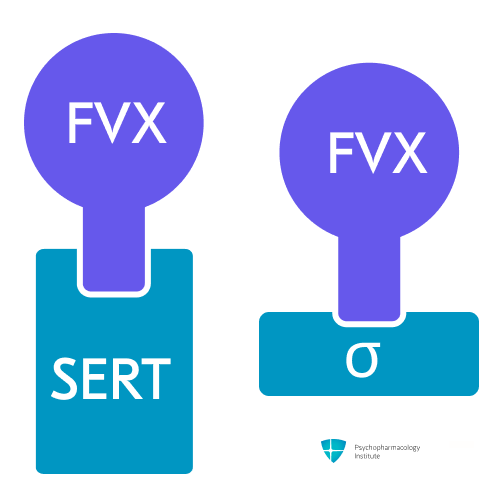
Fluvoxamine inhibits serotonin reuptake and has affinity for the sigma receptor
Pharmacokinetics
- Half-life: 16 hours.
- Fluvoxamine is metabolized by:
- CYP1A2 (minor)
- CYP2D6 (minor)
- Fluvoxamine inhibits these CYP450 isoenzymes:
- CYP1A2: Strong inhibitor
- Caution with: Warfarin, theophylline, propranolol, tizanidine
- CYP2C19: Moderate inhibitor
- Caution with: Omeprazole
- CYP2C9: Weak inhibitor
- CYP2D6: Weak inhibitor
- CYP3A4: Weak inhibitor
- CYP1A2: Strong inhibitor
- Avoid use with MAOIs (2-week washout if switching to an MAOI).
Dosage forms
- Tablets: 25 mg, 50 mg (scored), 100 mg (scored).
- ER capsules: 100 mg, 150 mg
Indications
FDA-approved indications and dosing
Obsessive-compulsive disorder
- Recommendations from 2022 WFSBP Guidelines:5
- Adults:
- Fluoxetine is recommended as a first-line SSRI along with escitalopram, fluoxetine, paroxetine, and sertraline.
- Children and adolescents:
- Fluvoxamine is one of the recommended first-line SSRIs, along with fluoxetine and sertraline.
- The evidence does not conclusively demonstrate that fluvoxamine is more effective than other SSRIs for OCD.
- Adults:
Adult dosing in OCD
| Tablets | Extended-Release Capsules | |
|---|---|---|
| Starting dose | 50 mg, at bedtime | 100 mg, at bedtime |
| Dose increases | 50 mg every 4–7 days as tolerated | Weekly increases of 50 mg, as tolerated to maximum effect |
| Maximum dose | 300 mg/day | 300 mg/day |
| Comments | Daily doses over 100 mg should be divided | – |
Children and adolescents (8–17 years) dosing
| Tablets | Extended-Release Capsules | |
|---|---|---|
| Starting dose | 25 mg, at bedtime | N/A |
| Dose increases | 25 mg every 4–7 days as tolerated | N/A |
| Maximum dose: 8–11 years | 200 mg/day | N/A |
| Maximum dose: 12–17 years | 300 mg/day | N/A |
| Notes | Daily doses over 50 mg should be divided | The lowest available dose of the ER capsules may not be appropriate for pediatric patients naïve to fluvoxamine maleate |
Off-label uses
Major depressive disorder
- Fluvoxamine is approved for treating depression in the UK but not in the U.S.
- Recommended dosing for depression:
- Starting dose: 50 mg–100 mg/day, in the evening.
- Maximum dose: 300 mg/day.
- Doses over 150 mg/day are given in divided doses.
Panic disorder
- Fluvoxamine is efficacious for panic disorder but has a higher risk of adverse events than other SSRIs.6
- Sertraline and escitalopram offer the best risk-benefit ratio.
Social anxiety disorder
- Fluvoxamine is efficacious for the treatment of social anxiety disorder, but it showed higher dropout rates from adverse events compared with other SSRIs.7
- Paroxetine has been suggested as a first-line treatment of SAD.8
Adjunctive to clozapine treatment
- Low-dose fluvoxamine (25 mg–50 mg/day) may reduce some metabolic side effects of clozapine treatment, such as weight gain.
- Fluvoxamine increases the clozapine/norclozapine ratio, by:
- Increasing clozapine plasma levels by 120%.9
- Decreasing norclozapine plasma levels.
- By increasing the ratio:
- Clozapine half-life is increased.
- There are more stable plasma levels and receptor occupancies.
- There is a decline in clozapine plasma levels.
- The use of adjunctive fluvoxamine might increase clozapine plasma levels in an unpredictable manner.
- Monitor clozapine plasma levels rigorously after initiating adjunctive fluvoxamine.10
- Adjunctive fluvoxamine should be considered in patients not responding optimally to clozapine when it is difficult to achieve plasma levels above 350 ng/mL–420 ng/mL.
- The evidence for fluvoxamine as an adjunctive treatment is too limited to make firm clinical recommendations.11
Adverse effects
Most common side effects
- Nausea
- Vomiting
- Somnolence
- Insomnia
- Sexual side effects
- Incidence in men:
- Abnormal ejaculation: 8%
- Impotence: 2%
- Incidence in women and men:
- Decreased libido: 2%
- Anorgasmia: 2%
- Incidence in men:
- Antidepressant-induced excessive sweating (ADIES):12 ,13
- Dose-related side effect.
- If antidepressant dose reduction is clinically feasible, it should be tried.
- Switching to another antidepressant might be effective.
- Adding another medication (an “antidote”):
- When antidepressant dose reduction or switching is not an option.
- Evidence of fair quality supports the use of terazosin (an alpha-1 adrenergic blocker)
- Tremor
- Headache
- Discontinuation syndrome
Serious side effects
- Hyponatremia
- May occur with SSRIs and SNRIs.
- The elderly may be at increased risk.
- Bleeding
- Aspirin use, NSAIDs, and warfarin may add to this risk.
Use in special populations
Breastfeeding
- A safety scoring system finds fluvoxamine use to be possible during breastfeeding.16
- Doses of up to 300 mg/day produce low levels in breast milk.
Hepatic impairment
- In patients with liver dysfunction, fluvoxamine clearance is decreased by approximately 30%.
- Start fluvoxamine at a low dose and increase it slowly with careful monitoring.
Renal impairment
- GFR 10–50mL/min:
- Dose as normal renal function.
- GFR <10mL/min:
- Start on a low dose and titrate slowly.
- Dose as for normal renal function.
Brand names
- US: Luvox
- Other brand names (some may be discontinued): Amvoxa, Anwu, Avoxin, Cererest, Deprivox, Depromel, Desifluvoxamin, Dumirox, Dumyrox, Faverin, Favoxil, Felixsan, Fevarin, Flox-ex, Floxyfral, Floxyfral bruchrille, Floxyfral m bruchrille, Floxyfral ohne bruchrill, Floxyfral ohne bruchrille, Fluvamteva, Fluvaris, Fluvator, Fluvo, Fluvohexal, Fluvoprex, Fluvosol, Fluvox, Fluvoxadura, Fluvoxamin Ratiopharm, Fluvoxamin Synthon, Fluvoxamin Teva, Fluvoxamin al, Fluvoxamin beta, Fluvoxamin stada, Fluvoxamina EG, Fluvoxamina Generis, Fluvoxamina Sandoz, Fluvoxin, Fluxamine, Forezol, Frext, Fulvana, Lote, Maveral, Movox, Ocdox, Oxamine, Psyvoxin, Relafin, Revilife, Revoc, Revoxin, Rokona, Sorest, Statomain, Uvox, Voxaday, Voxam, Voxamin, Voxamine, Voxidep, Vuminix
References
- Actavis Pharma, Inc. (2022). Fluvoxamine maleate: Prescribing Information. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8bbd7e39-b9ab-4716-9522-aa8c4b92210e.↩︎
- Hindmarch, I., & Hashimoto, K. (2010). Cognition and depression: The effects of fluvoxamine, a sigma-1 receptor agonist, reconsidered. Human Psychopharmacology: Clinical and Experimental, 25(3), 193–200. https://doi.org/10.1002/hup.1106.↩︎
- Albayrak, Y., & Hashimoto, K. (2017). Sigma-1 Receptor Agonists and Their Clinical Implications in Neuropsychiatric Disorders. In S. B. Smith & T.-P. Su (Eds.), Sigma Receptors: Their Role in Disease and as Therapeutic Targets (Vol. 964, pp. 153–161). Springer International Publishing. https://doi.org/10.1007/978-3-319-50174-1_11.↩︎
- British National Formulary. (2023, September 14). Fluvoxamine maleate. https://bnf.nice.org.uk/drugs/fluvoxamine-maleate/.↩︎
- Bandelow, B., Allgulander, C., Baldwin, D. S., Costa, D. L. D. C., Denys, D., Dilbaz, N., Domschke, K., Eriksson, E., Fineberg, N. A., Hättenschwiler, J., Hollander, E., Kaiya, H., Karavaeva, T., Kasper, S., Katzman, M., Kim, Y.-K., Inoue, T., Lim, L., Masdrakis, V., … Zohar, J. (2023). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders – Version 3. Part I: Anxiety disorders. The World Journal of Biological Psychiatry, 24(2), 79–117. https://doi.org/10.1080/15622975.2022.2086295.↩︎
- Chawla, N., Anothaisintawee, T., Charoenrungrueangchai, K., Thaipisuttikul, P., McKay, G. J., Attia, J., & Thakkinstian, A. (2022). Drug treatment for panic disorder with or without agoraphobia: Systematic review and network meta-analysis of randomised controlled trials. BMJ (Clinical Research Ed.), 376. https://doi.org/10.1136/bmj-2021-066084.↩︎
- Liu, X., Li, X., Zhang, C., Sun, M., Sun, Z., Xu, Y., & Tian, X. (2018). Efficacy and tolerability of fluvoxamine in adults with social anxiety disorder: A meta-analysis. Medicine, 97(28). https://doi.org/10.1097/MD.0000000000011547.↩︎
- Williams, T., McCaul, M., Schwarzer, G., Cipriani, A., Stein, D. J., & Ipser, J. (2020). Pharmacological treatments for social anxiety disorder in adults: A systematic review and network meta-analysis. Acta Neuropsychiatrica, 32(4), 169–176. https://doi.org/10.1017/neu.2020.6.↩︎
- Lu, M. L., Lane, H. Y., Chen, K. P., Jann, M. W., Su, M. H., & Chang, W. H. (2000). Fluvoxamine reduces the clozapine dosage needed in refractory schizophrenic patients. The Journal of Clinical Psychiatry, 61(8), 594–599. https://doi.org/10.4088/jcp.v61n0809.↩︎
- Gee, S., & Howes, O. (2016). Optimising treatment of schizophrenia: The role of adjunctive fluvoxamine. Psychopharmacology, 233(5), 739–740. https://doi.org/10.1007/s00213-016-4207-z.↩︎
- Polcwiartek, C., & Nielsen, J. (2016). The clinical potentials of adjunctive fluvoxamine to clozapine treatment: A systematic review. Psychopharmacology, 233(5), 741–750. https://doi.org/10.1007/s00213-015-4161-1.↩︎
- Thompson, S., Compton, L., Chen, J.-L., & Fang, M.-L. (2021). Pharmacologic treatment of antidepressant-induced excessive sweating: A systematic review. Archives of Clinical Psychiatry (São Paulo), 48, 57–65. https://doi.org/10.15761/0101-60830000000279.↩︎
- Mago, R. (2023, September 6). Antidepressant-induced excessive sweating (ADIES) or hyperhidrosis: Management. https://simpleandpractical.com/antidepressant-induced-excessive-sweating-adies-hyperhidrosis-management/.↩︎
- Puzantian, T., & Carlat, D. J. (2020). Medication fact book for psychiatric practice (Fifth edition). Carlat Publishing, LLC.↩︎
- Apotex Corp. (n.d.). Fluvoxamine maleate: Highlights of prescribing information. Retrieved September 8, 2023, from https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=6eeb14df-6fcf-a737-5359-5744eb4accea.↩︎
- Drugs and Lactation Database (LactMed®). (2023, September 1). Fluvoxamine. Bethesda (MD): National Institute of Child Health and Human Development; 2006-. [Updated 2023 May 15]. https://www.ncbi.nlm.nih.gov/books/NBK501187/.↩︎



