In a nutshell
Esketamine is an NMDA receptor antagonist delivered via intranasal administration for treatment-resistant depression and major depression with suicidal ideation. Its primary clinical advantage is a rapid onset of antidepressant effect (within 24 hours), faster than traditional antidepressants, although long-term efficacy remains unclear. Due to risks of dissociation, sedation, blood pressure elevation, and potential for misuse, esketamine requires specialized administration and monitoring through the REMS program.

- When to consider adding esketamine:
- When rapid response is needed (24-hour onset vs. weeks for traditional antidepressants)
- For treatment-resistant depression after failure of ≥2 adequate antidepressant trials
- As a “response accelerator” in the acute phase, while waiting for oral antidepressants to take effect
- When standard antidepressants haven’t been tolerated or have failed
- Esketamine may not be appropriate when:
- Patient cannot attend required in-office treatment sessions with monitoring
- Cardiovascular risk factors (especially uncontrolled hypertension)
- History of psychosis or substance use disorders
- Respiratory compromise
- Cost or accessibility concerns (requires REMS-certified facility)
- Long-term maintenance is the primary goal (limited long-term data)
Pharmacodynamics and mechanism of action
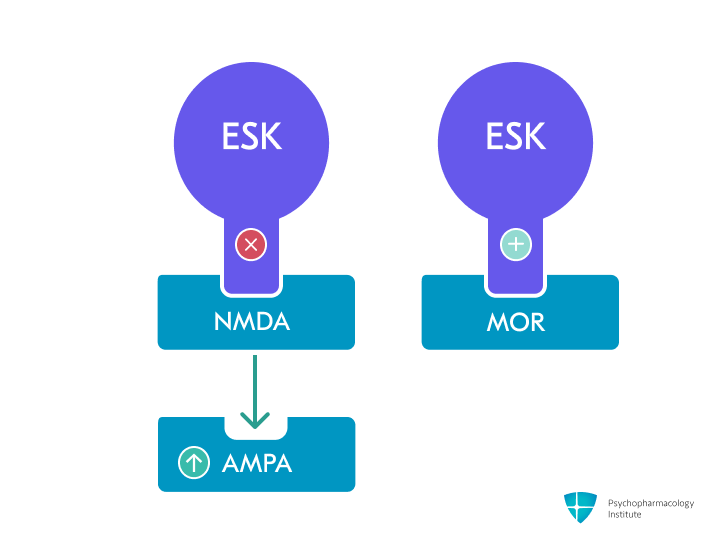
- Primary mechanism: Non-competitive, nonselective NMDA receptor antagonist [1,2]
- Esketamine (S-enantiomer) has 2-3 fold higher NMDA receptor affinity than R-ketamine [2,3]
- Binds to the phencyclidine (PCP) binding site within the NMDA receptor channel pore, blocking glutamate-induced calcium influx [4]
- Preferentially inhibits NMDA receptors on GABAergic interneurons, leading to disinhibition and increased excitatory neuron firing [5]
- AMPA receptor modulation
- Indirectly enhances AMPA receptor signaling by blocking NMDA receptors, with this glutamate signaling shift potentially critical for rapid antidepressant effects [5–7]
- Downstream effects on brain plasticity
- Increases BDNF release, potentially explaining rapid synaptogenic and antidepressant effects [5,6]
- Activates mTOR signaling pathway, leading to increased protein synthesis and dendritic spine formation in prefrontal cortex [8,9]
- These neuroplasticity effects contrast with conventional antidepressants, which require weeks for similar adaptations [9]
- Secondary pharmacological targets: Opioid receptors
- Binds to mu (μ, MOR: mu opioid receptors), kappa (κ), and delta (δ) opioid receptors with low affinity (10-20 fold weaker than to NMDA receptors) [5,6]
- Affinity for kappa and delta receptors is generally even weaker
- The role of opioid interactions in esketamine’s antidepressant effects remains debated, with conflicting evidence from naltrexone studies and genetic analyses [5,7,10]
- Binds to mu (μ, MOR: mu opioid receptors), kappa (κ), and delta (δ) opioid receptors with low affinity (10-20 fold weaker than to NMDA receptors) [5,6]
- Additional receptor interactions
- Weak inhibitory activity at monoamine transporters (SERT, NET, DAT) [5,11]
- Weak agonist activity at dopamine D2 receptors and antagonist activity at serotonin 5-HT3 receptors [11]
Pharmacokinetics
Metabolism
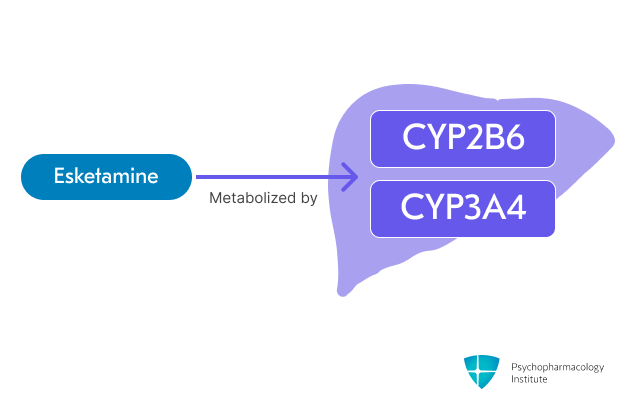
- Esketamine is primarily metabolized through oxidation via CYP2B6 and CYP3A4 [1,12]
- Minor pathways involve CYP2C19 and CYP2C9
- Esketamine levels potentially increased by:
- CYP3A4 inhibitors
- Clarithromycin, ketoconazole, itraconazole
- CYP2B6 inhibitors
- Ticlopidine
- CYP3A4 inhibitors
- Esketamine levels potentially decreased by:
- CYP3A4 inducers
- Carbamazepine, rifampin, St. John’s Wort.
- CYP3A4 inducers
- However, dose adjustment of esketamine is not warranted in patients taking an inhibitor of CYP2B6 or CYP3A4, an inducer of CYP3A4 or CYP2B6 [13]
- Esketamine is not expected to significantly alter the metabolism of other drugs by inhibiting or inducing CYP enzymes in standard antidepressant doses [1,14]
- Drug interactions:
- CNS depressants
- Concomitant use may increase sedation
- Close monitoring is recommended when used with CNS depressants (benzodiazepines, opioids, alcohol) [1]
- Psychostimulants and MAOIs
- May enhance hypertensive effects
- Close blood pressure monitoring is required with concurrent psychostimulant or MAOI use [1]
- Nasal corticosteroids or decongestants
- May diminish therapeutic effect of intranasal esketamine
- Administer at least 1 hour before esketamine on treatment days [1]
- CNS depressants
- Note: Esketamine can trigger false-positive methadone results in urine immunoassays. Confirmatory testing is recommended for positive screens [1]
Half-life
- Esketamine
- Elimination half-life of approximately 7-12 hours, increased in moderate hepatic impairment [1]
- Time to maximum concentration (Tmax): 20-40 minutes after administration [1]
- Noresketamine (active metabolite) half-life is approximately 8 hours [5]
Dosage forms
Dosage forms
- Intranasal spray device (Spravato)
- 28 mg per device (delivers two sprays of 14 mg each)
Logistical aspects
- Requires REMS program certification for dispensing
- Must be administered under direct healthcare professional supervision
- Device practicalities:
- Do not prime the device before use
- Use 2 devices (for a 56 mg dose) or 3 devices (for an 84 mg dose), with a 5-minute rest between the use of each device
- Patient preparation [1]
- Avoid food for at least 2 hours before administration
- Avoid drinking liquids at least 30 minutes before administration
- Administer nasal corticosteroids or decongestants (if needed) at least 1 hour before Esketamine
- Patient must not drive on the day of administration
- Administration monitoring [1]
- Assess blood pressure before dosing
- Reassess blood pressure approximately 40 minutes post-dose
- Monitor respiratory status (including pulse oximetry) for at least 2 hours
- Patient may be discharged only if clinically stable for at least two hours post-dose
Indications
FDA-Approved Indications
Treatment-resistant depression (TRD)
- Esketamine is indicated for TRD in adults who have failed to respond to at least two different trials of antidepressants of adequate dose and duration in the current depressive episode [1,15]
- Approved for use both as monotherapy or in conjunction with an oral antidepressant [1]
- Second-line treatment option for adjunctive medication for Difficult to Treat Depression, according to latest CANMAT guidelines [16]
- Onset of action:
- Rapid onset of action within 24 hours, with clinical improvement occurring faster than traditional antidepressants [17,18]
- Significant but modest early effect (24 hours): Effect size 0.33 [19]
- Some authors suggest esketamine may function as a response accelerator.
- It could be used initially to achieve faster improvement and then discontinued once a response is achieved [19]
- Long-term efficacy:
- Long-term efficacy remains inconclusive, with evidence of diminishing effects over time [17,19,20]
- Short-to-medium term findings (Meta-analysis evidence)
- Progressive decline in effect sizes: 0.33 at 24 hours → 0.25 at week 1 → 0.15 at week 2 → 0.23 at week 4 [19]
- Most trials (5 of 6) were negative at week 4 for primary outcomes [19]
- SUSTAIN-1 maintenance trial showed high relapse rates after discontinuation, with potential withdrawal effects contributing to relapse [19]
- Long-term extension data (SUSTAIN-3 study):
- 49-50% remission rates at 2 years (5+ years follow-up, 3,777 patient-years) [21]
- Practical considerations:
- Most patients (75% of visits) require frequent dosing (weekly or every 2 weeks)
- Raises questions about long-term sustainability and feasibility
- Short-to-medium term findings (Meta-analysis evidence)
- Long-term efficacy remains inconclusive, with evidence of diminishing effects over time [17,19,20]
- Dosing:
- Induction phase (weeks 1-4)
- Starting dose: 56 mg twice weekly
- May increase to 84 mg twice weekly based on efficacy and tolerability
- Maintenance phase (weeks 5 and beyond)
- Week 5-8: 56 mg or 84 mg once weekly
- Week 9 and beyond: 56 mg or 84 mg once weekly or once every 2 weeks
- Use the lowest effective frequency to maintain response or remission [1]
- Assess therapeutic benefit periodically to determine the need for continued treatment
- Induction phase (weeks 1-4)
Major depressive disorder (MDD) with acute suicidal ideation or behavior
- Indicated for the rapid reduction of depressive symptoms in adults with MDD who have suicidal ideation with intent [1,22]
- Used in conjunction with an oral antidepressant
- May provide acute symptom relief while conventional antidepressants take effect [17]
- Not a substitute for hospitalization when indicated [1]
- Conflicting evidence:
- A recent systematic review and meta-analysis found no significant effect on suicidality at any time point (effect size 0.10 at days 2-5, 0.04 at week 4), calling into question this indication [19]
- Dosing:
- Starting dose:
- 84 mg twice weekly for 4 weeks
- Dosage may be reduced to 56 mg twice weekly based on tolerability
- Reassess to determine the need for continued esketamine treatment.
- Its use beyond 4 weeks has not been systematically evaluated for this indication [1]
- Starting dose:
Off-label Uses
Bipolar depression
- Not recommended for routine use in bipolar depression
- Several systematic reviews report promising results for racemic ketamine, with response rates of 48–55% and remission rates of 30–50% [23,24]
- Esketamine-specific data remains sparse, as most systematic reviews do not separate outcomes by compound
- Caution due to theoretical risk of mood switching in bipolar patients [23,24]
Side Effects
Most common side effects
Neurological/Psychiatric
- Dissociation (28% TRD, 48% MDD with suicidal ideation)
- Most common adverse effect and primary reason for treatment discontinuation in MDD-with suicidal ideation [1,15]
- May involve transient perceptual changes, depersonalization, and derealization.
- Typically begins shortly after administration and resolves within 2 hours; attenuated after repeated administrations [15,25]
- Higher rates reported using structured scales in clinical assessments [1]
- Dizziness (22% TRD, 45% MDD with suicidal ideation) [1,26]
- Sedation/somnolence (6-29% incidence)) [1]
- Monitoring for at least two hours post-administration is recommended, due to the risk of delayed or prolonged sedation
- 0.3% to 0.4% of esketamine-treated patients may experience loss of consciousness
- Patients should not drive or operate machinery until the next day after restful sleep [1]
- Higher rates reported using structured scales in clinical assessments [1]
- Headache (19% incidence)
- Dysgeusia (altered taste) (4-20% incidence)
- Anxiety (10-15% incidence)
- Cognitive impairment (11-13% incidence) [26]
- Temporary cognitive deficits can occur, typically observed within the first 40 minutes post-dose, returning to baseline within approximately 2 hours.
- Long-term effects on cognitive function with repeated use are not fully characterized.
- Cognitive functioning remained stable or slightly improved over extended treatment periods in 1-year and 3-year open-label clinical trials [25,27]
Cardiovascular
- Blood pressure increase (5%; including hypertension)
- Characteristics
- Transient, dose-dependent elevations peaking at 40 minutes post-dose, persisting ~4 hours.
- Typical increases: 7-10 mmHg systolic, 4-6 mmHg diastolic [1]
- 3-19% of patients experience substantial increases (≥40 mmHg systolic and/or ≥25 mmHg diastolic) within the first 1.5 hours [1]
- Substantial increases may occur even if smaller effects observed with previous doses [1]
- Blood pressure monitoring required
- Assess BP prior to each administration
- Monitor for at least 2 hours post-dose, with measurement around 40 minutes post-dose
- Continue monitoring until values decline to acceptable levels [1]
- Hypertensive crisis
- Rare but potentially severe elevations in blood pressure may require emergency intervention
- Contraindicated in patients for whom BP increases pose serious risks
- Enhanced monitoring recommended for patients with a history of hypertensive encephalopathy
- Requires special attention with concomitant psychostimulants or MAOIs [1]
- Consider delaying treatment in patients with elevated BP (>140/90 mmHg)
- Use with caution in patients with other cardiovascular or cerebrovascular conditions
- Characteristics
Gastrointestinal
- Nausea (25-32% incidence) [1]
- Most common gastrointestinal side effect
- Generally transient, resolving the same day with a median duration of less than 1 hour
- Can be minimized by fasting for at least 2 hours before administration [1]
- Vomiting (6-12% incidence) [1]
- Also, it typically resolves the same day
Other common side effects
- Vertigo (3-23% incidence)
- Hypoesthesia: (4%-13% incidence)
- Fatigue (4-11% incidence)
- Feeling drunk (4-7% incidence)
- Urinary tract symptoms (pollakiuria, dysuria, micturition urgency, nocturia) (2-3% incidence) [1]
Severe side effects
- Respiratory depression
- Rare but serious risk, including reports of respiratory arrest [1]
- Monitoring required: Respiratory status and pulse oximetry for ≥2 hours post-dose; discharge only after confirming clinical stability [1]
- Increased risk: Concomitant CNS depressants and compromised respiratory function [1]
- Ulcerative/intersticial cystitits
- Cases reported with long-term off-label ketamine use, rare with therapeutic esketamine (1.1% of postmarketing AEs) [28–30]
- Regular monitoring of urinary symptoms and periodic urinalysis recommended [16]
- Suicidal thoughts and behaviors
- Recent meta-analysis raised concerns about deaths and emerging suicidality during esketamine trials [19]
- SUSTAIN-3 long-term data: 9 deaths over 5 years (including 1 suicide), all deemed unrelated to esketamine by investigators [21]
- Enhanced monitoring is recommended for all patients, especially during early treatment and dose adjustments [1]
- Abuse and dependence
- Schedule III controlled substance due to abuse potential
- Physical and psychological dependence may occur [1,31]
- Despite concerns based on ketamine’s history [32], a recent literature review suggests minimal actual risk of misuse in therapeutic settings [33]
- Careful patient selection and monitoring required, particularly in patients with a history of substance use disorders
Contraindications
- Aneurysmal vascular disease (including thoracic and abdominal aorta, intracranial, and peripheral arterial vessels)
- Arteriovenous malformation
- History of intracerebral hemorrhage
- Known hypersensitivity or allergic reaction to esketamine, ketamine, or formulation ingredients [1]
Use in special populations
Pregnancy
- Not recommended: Animal studies show potential fetal harm, with limited human data available for psychiatric use [1]
- Fetal brain development may be affected, particularly in the third trimester, based on published ketamine findings and NMDA receptor antagonist effects, with preclinical studies showing developmental delays and neurobehavioral impairments [1,34]
- Limited data from cesarean section analgesia studies provide insufficient conclusions about neonatal effects [35,36]
Breastfeeding
- Esketamine is present in human breast milk [1]
- The manufacturer does not recommend breastfeeding during treatment since NMDA receptor antagonism affects rapid brain development in infants [1]
- The window of vulnerability to neurodevelopmental effects may extend to approximately 3 years of age [1]
Hepatic impairment
- Mild to moderate impairment (Child-Pugh Class A and B)
- There are no dosage adjustments provided in the manufacturer’s labeling (esketamine has not been studied).
- Patients with moderate impairment may need monitoring for adverse effects for a longer duration after administration [1]
- Severe impairment (Child-Pugh Class C)
- Use not recommended; not studied in this population [1]
Renal impairment
- No dosage adjustments provided in the manufacturer’s labeling
- Limited study data available for patients with renal impairment [1]
Elderly
- Efficacy in geriatric treatment-resistant depression (TRD) not statistically superior to placebo at 4 weeks [1,37]
- Consider using lower initial doses (28 mg twice weekly for 4 weeks) [37,38]
Brand names
* US: Spravato – Canada: Spravato – Other countries/regions: Spravato
References
- Food, U. S., & Administration, D. (2025). SPRAVATO® esketamine hydrochloride solution Janssen Pharmaceuticals Inc. Prescribing Information.
- Vasiliu, O. (2023). Esketamine for treatment‑resistant depression: A review of clinical evidence (Review). Experimental and Therapeutic Medicine, 25(3), 111. https://doi.org/10.3892/etm.2023.11810
- Liu, P., Zhang, S.-S., Liang, Y., Gao, Z.-J., Gao, W., & Dong, B.-H. (2022). Efficacy and Safety of Esketamine Combined with Antidepressants for Treatment-Resistant Depression: A Meta-Analysis. Neuropsychiatric Disease and Treatment, 18, 2855–2865. https://doi.org/10.2147/NDT.S388764
- Ye, S., Han, Y., Wei, Z., & Li, J. (2023). Binding Affinity and Mechanisms of Potential Antidepressants Targeting Human NMDA Receptors. Molecules, 28(11), 4346. https://doi.org/10.3390/molecules28114346
- McIntyre, R. S., Rosenblat, J. D., Nemeroff, C. B., Sanacora, G., Murrough, J. W., Berk, M., Brietzke, E., Dodd, S., Gorwood, P., Ho, R., Iosifescu, D. V., Lopez Jaramillo, C., Kasper, S., Kratiuk, K., Lee, J. G., Lee, Y., Lui, L. M. W., Mansur, R. B., Papakostas, G. I., … Stahl, S. (2021). Synthesizing the Evidence for Ketamine and Esketamine in Treatment-Resistant Depression: An International Expert Opinion on the Available Evidence and Implementation. American Journal of Psychiatry, 178(5), 383–399. https://doi.org/10.1176/appi.ajp.2020.20081251
- Zanos, P., Moaddel, R., Morris, P. J., Riggs, L. M., Highland, J. N., Georgiou, P., Pereira, E. F. R., Albuquerque, E. X., Thomas, C. J., Zarate, C. A., & Gould, T. D. (2018). Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacological Reviews, 70(3), 621–660. https://doi.org/10.1124/pr.117.015198
- Williams, N. R., Heifets, B. D., Blasey, C., Sudheimer, K., Pannu, J., Pankow, H., Hawkins, J., Birnbaum, J., Lyons, D. M., Rodriguez, C. I., & Schatzberg, A. F. (2018). Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism. American Journal of Psychiatry, 175(12), 1205–1215. https://doi.org/10.1176/appi.ajp.2018.18020138
- K v, A., Mohan, A. S., & Chakravarty, S. (2020). Rapid acting antidepressants in the mTOR pathway: Current evidence. Brain Research Bulletin, 163, 170–177. https://doi.org/10.1016/j.brainresbull.2020.07.022
- Duman, R. S., Aghajanian, G. K., Sanacora, G., & Krystal, J. H. (2016). Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nature Medicine, 22(3), 238–249. https://doi.org/10.1038/nm.4050
- Sanacora, G. (2019). Caution Against Overinterpreting Opiate Receptor Stimulation as Mediating Antidepressant Effects of Ketamine. The American Journal of Psychiatry, 176(3), 249. https://doi.org/10.1176/appi.ajp.2018.18091061
- Veraart, J. K. E., Smith-Apeldoorn, S. Y., Bakker, I. M., Visser, B. A. E., Kamphuis, J., Schoevers, R. A., & Touw, D. J. (2021). Pharmacodynamic Interactions Between Ketamine and Psychiatric Medications Used in the Treatment of Depression: A Systematic Review. International Journal of Neuropsychopharmacology, 24(10), 808–831. https://doi.org/10.1093/ijnp/pyab039
- McIntyre, R. S., Alsuwaidan, M., Baune, B. T., Berk, M., Demyttenaere, K., Goldberg, J. F., Gorwood, P., Ho, R., Kasper, S., Kennedy, S. H., Ly-Uson, J., Mansur, R. B., McAllister-Williams, R. H., Murrough, J. W., Nemeroff, C. B., Nierenberg, A. A., Rosenblat, J. D., Sanacora, G., Schatzberg, A. F., … Maj, M. (2023). Treatment-resistant depression: Definition, prevalence, detection, management, and investigational interventions. World Psychiatry: Official Journal of the World Psychiatric Association (WPA), 22(3), 394–412. https://doi.org/10.1002/wps.21120
- Drug Evaluation and Research., C. for. (n.d.). Clinical Pharmacology and Biopharmaceutics Review(s). Retrieved April 23, 2025, from https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211243Orig1s000ClinPharmR.pdf
- Bahr, R., Lopez, A., & Rey, J. A. (2019). Intranasal Esketamine (SpravatoTM) for Use in Treatment-Resistant Depression In Conjunction With an Oral Antidepressant. Pharmacy and Therapeutics, 44(6), 340–375. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6534172/
- Popova, V., Daly, E. J., Trivedi, M., Cooper, K., Lane, R., Lim, P., Mazzucco, C., Hough, D., Thase, M. E., Shelton, R. C., Molero, P., Vieta, E., Bajbouj, M., Manji, H., Drevets, W. C., & Singh, J. B. (2019). Efficacy and Safety of Flexibly Dosed Esketamine Nasal Spray Combined With a Newly Initiated Oral Antidepressant in Treatment-Resistant Depression: A Randomized Double-Blind Active-Controlled Study. The American Journal of Psychiatry, 176(6), 428–438. https://doi.org/10.1176/appi.ajp.2019.19020172
- Lam, R. W., Kennedy, S. H., Adams, C., Bahji, A., Beaulieu, S., Bhat, V., Blier, P., Blumberger, D. M., Brietzke, E., Chakrabarty, T., Do, A., Frey, B. N., Giacobbe, P., Gratzer, D., Grigoriadis, S., Habert, J., Ishrat Husain, M., Ismail, Z., McGirr, A., … Milev, R. V. (2024). Canadian Network for Mood and Anxiety Treatments (CANMAT) 2023 Update on Clinical Guidelines for Management of Major Depressive Disorder in Adults: Réseau canadien pour les traitements de l’humeur et de l’anxiété (CANMAT) 2023 : Mise à jour des lignes directrices cliniques pour la prise en charge du trouble dépressif majeur chez les adultes. Can. J. Psychiatry, 69(9), 641–687. https://doi.org/10.1177/07067437241245384
- Oraee, S., Alinejadfard, M., Golsorkh, H., Sadeghian, M., Fanaei, M., Centis, R., D’Ambrosio, L., Sotgiu, G., Goudarzi, H., Migliori, G. B., & Nasiri, M. J. (2024). Intranasal esketamine for patients with major depressive disorder: A systematic review and meta-analysis. Journal of Psychiatric Research, 180, 371–379. https://doi.org/10.1016/j.jpsychires.2024.11.010
- Bahji, A., Zarate, C. A., & Vazquez, G. H. (2022). EFFICACY AND SAFETY OF RACEMIC KETAMINE AND ESKETAMINE FOR DEPRESSION: A SYSTEMATIC REVIEW AND META-ANALYSIS. Expert Opinion on Drug Safety, 21(6), 853–866. https://doi.org/10.1080/14740338.2022.2047928
- Fountoulakis, K. N., Saitis, A., & Schatzberg, A. F. (2025). Esketamine Treatment for Depression in Adults: A PRISMA Systematic Review and Meta-Analysis. American Journal of Psychiatry. https://doi.org/10.1176/appi.ajp.20240515
- Lima, T. de M., Visacri, M. B., & Aguiar, P. M. (2022). Use of ketamine and esketamine for depression: An overview of systematic reviews with meta-analyses. European Journal of Clinical Pharmacology, 78(3), 311–338. https://doi.org/10.1007/s00228-021-03216-8
- Zaki, N., Chen, L. (Nancy)., Lane, R., Doherty, T., Drevets, W. C., Morrison, R. L., Sanacora, G., Wilkinson, S. T., Young, A. H., Lacerda, A. L. T., Paik, J.-W., Popova, V., & Fu, D.-J. (2025). Safety and Efficacy with Esketamine in Treatment-Resistant Depression: Long-Term Extension Study. International Journal of Neuropsychopharmacology, pyaf027. https://doi.org/10.1093/ijnp/pyaf027
- Ionescu, D. F., Fu, D.-J., Qiu, X., Lane, R., Lim, P., Kasper, S., Hough, D., Drevets, W. C., Manji, H., & Canuso, C. M. (2020). Esketamine Nasal Spray for Rapid Reduction of Depressive Symptoms in Patients With Major Depressive Disorder Who Have Active Suicide Ideation With Intent: Results of a Phase 3, Double-Blind, Randomized Study (ASPIRE II). International Journal of Neuropsychopharmacology, 24(1), 22–31. https://doi.org/10.1093/ijnp/pyaa068
- Fancy, F., Haikazian, S., Johnson, D. E., Chen-Li, D. C. J., Levinta, A., Husain, M. I., Mansur, R. B., & Rosenblat, J. D. (2023). Ketamine for bipolar depression: An updated systematic review. Therapeutic Advances in Psychopharmacology, 13, 20451253231202723. https://doi.org/10.1177/20451253231202723
- Nunez, N. A., Joseph, B., Kumar, R., Douka, I., Miola, A., Prokop, L. J., Mickey, B. J., & Singh, B. (2023). An Update on the Efficacy of Single and Serial Intravenous Ketamine Infusions and Esketamine for Bipolar Depression: A Systematic Review and Meta-Analysis. Brain Sciences, 13(12, 12), 1672. https://doi.org/10.3390/brainsci13121672
- Wajs, E., Aluisio, L., Holder, R., Daly, E. J., Lane, R., Lim, P., George, J. E., Morrison, R. L., Sanacora, G., Young, A. H., Kasper, S., Sulaiman, A. H., Li, C.-T., Paik, J.-W., Manji, H., Hough, D., Grunfeld, J., Jeon, H. J., Wilkinson, S. T., … Singh, J. B. (2020). Esketamine Nasal Spray Plus Oral Antidepressant in Patients With Treatment-Resistant Depression: Assessment of Long-Term Safety in a Phase 3, Open-Label Study (SUSTAIN-2). The Journal of Clinical Psychiatry, 81(3), 19m12891. https://doi.org/10.4088/JCP.19m12891
- Fedgchin, M., Trivedi, M., Daly, E. J., Melkote, R., Lane, R., Lim, P., Vitagliano, D., Blier, P., Fava, M., Liebowitz, M., Ravindran, A., Gaillard, R., Ameele, H. V. D., Preskorn, S., Manji, H., Hough, D., Drevets, W. C., & Singh, J. B. (2019). Efficacy and Safety of Fixed-Dose Esketamine Nasal Spray Combined With a New Oral Antidepressant in Treatment-Resistant Depression: Results of a Randomized, Double-Blind, Active-Controlled Study (TRANSFORM-1). International Journal of Neuropsychopharmacology, 22(10), 616–630. https://doi.org/10.1093/ijnp/pyz039
- Zaki, N., Chen, L. (Nancy)., Lane, R., Doherty, T., Drevets, W. C., Morrison, R. L., Sanacora, G., Wilkinson, S. T., Popova, V., & Fu, D.-J. (2023). Long-term safety and maintenance of response with esketamine nasal spray in participants with treatment-resistant depression: Interim results of the SUSTAIN-3 study. Neuropsychopharmacology, 48(8), 1225–1233. https://doi.org/10.1038/s41386-023-01577-5
- Morgan, C. J. A., Curran, H. V., & Independent Scientific Committee on Drugs. (2012). Ketamine use: A review. Addiction (Abingdon, England), 107(1), 27–38. https://doi.org/10.1111/j.1360-0443.2011.03576.x
- Samalin, L., Rothärmel, M., Mekaoui, L., Gaudré-Wattinne, E., Codet, M.-A., Bouju, S., & Sauvaget, A. (2022). Esketamine nasal spray in patients with treatment-resistant depression: The real-world experience in the French cohort early-access programme. International Journal of Psychiatry in Clinical Practice, 26(4), 352–362. https://doi.org/10.1080/13651501.2022.2030757
- Liu, R., Liu, C., Feng, D., Guo, T., & Wang, Y. (2024). Pharmacovigilance of esketamine nasal spray: An analysis of the FDA adverse event reporting system database. Frontiers in Pharmacology, 15. https://doi.org/10.3389/fphar.2024.1414703
- Orsolini, L., Salvi, & and Volpe, U. (2022). Craving and addictive potential of esketamine as side effects? Expert Opinion on Drug Safety, 21(6), 803–812. https://doi.org/10.1080/14740338.2022.2071422
- Van Amsterdam, J., & Van Den Brink, W. (2022). Harm related to recreational ketamine use and its relevance for the clinical use of ketamine. A systematic review and comparison study. Expert Opinion on Drug Safety, 21(1), 83–94. https://doi.org/10.1080/14740338.2021.1949454
- Roncero, C., Merizalde-Torres, M., Szerman, N., Torrens, M., Vega, P., Andres-Olivera, P., & Javier Álvarez, F. (2025). Is there a risk of esketamine misuse in clinical practice? Therapeutic Advances in Drug Safety, 16, 20420986241310685. https://doi.org/10.1177/20420986241310685
- Huang, R., Lin, B., Tian, H., Luo, Q., & Li, Y. (2023). Prenatal Exposure to General Anesthesia Drug Esketamine Impaired Neurobehavior in Offspring. Cellular and Molecular Neurobiology, 43(6), 3005–3022. https://doi.org/10.1007/s10571-023-01354-4
- Wang, Y., Zhang, Q., Dai, X., Xiao, G., & Luo, H. (2022). Effect of low-dose esketamine on pain control and postpartum depression after cesarean section: A retrospective cohort study. Annals of Palliative Medicine, 11(1), 45–57. https://doi.org/10.21037/apm-21-3343
- Wang, S., Deng, C.-M., Zeng, Y., Chen, X.-Z., Li, A.-Y., Feng, S.-W., Xu, L.-L., Chen, L., Yuan, H.-M., Hu, H., Yang, T., Han, T., Zhang, H.-Y., Jiang, M., Sun, X.-Y., Guo, H.-N., Sessler, D. I., & Wang, D.-X. (2024). Efficacy of a single low dose of esketamine after childbirth for mothers with symptoms of prenatal depression: Randomised clinical trial. https://doi.org/10.1136/bmj-2023-078218
- Ochs-Ross, R., Wajs, E., Daly, E. J., Zhang, Y., Lane, R., Lim, P., Drevets, W. C., Steffens, D. C., Sanacora, G., Jamieson, C., Hough, D., Manji, H., & Singh, J. B. (2022). Comparison of Long-Term Efficacy and Safety of Esketamine Nasal Spray Plus Oral Antidepressant in Younger Versus Older Patients With Treatment-Resistant Depression: Post-Hoc Analysis of SUSTAIN-2, a Long-Term Open-Label Phase 3 Safety and Efficacy Study. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry, 30(5), 541–556. https://doi.org/10.1016/j.jagp.2021.09.014
- Depression in adults: Treatment and management. (n.d.).



