In a nutshell
Dextromethorphan/Bupropion (DXM/BUP) is the first oral NMDA receptor antagonist approved for major depressive disorder (MDD). Bupropion inhibits dextromethorphan’s metabolism, increasing its bioavailability. Its primary clinical advantage is a rapid onset of antidepressant effect, with symptom improvement seen as early as the first week of treatment. Its twice-daily extended-release tablet is convenient, but potent CYP2D6 inhibition, seizure risk, and abuse potential call for careful patient selection and monitoring.

- Dextromethorphan/Bupropion vs. Esketamine:
- Dextromethorphan/Bupropion targets moderate-to-severe MDD symptoms
- Esketamine is indicated for treatment-resistant depression (TRD)
- Initial trials with Dextromethorphan/Bupropion for TRD showed early promise but failed to maintain superiority at trial endpoints [1,2]
- Dextromethorphan/Bupropion may be an option when:
- Rapid antidepressant response is a priority
- Patients have inadequate responses to first-line treatments (e.g., SSRIs or SNRIs)
- Depression is accompanied by fatigue or anhedonia (leveraging the bupropion component)
- SSRI/SNRI-associated sexual dysfunction is a significant concern
- Prefer alternatives when:
- History of seizure or eating disorders (anorexia/bulimia), absolute contraindications
- Patient is undergoing abrupt discontinuation of alcohol, benzodiazepines, or sedatives
- High risk of drug-drug interactions (potent CYP2D6 inhibitor)
- Cost is a significant barrier
- Patient is pregnant or breastfeeding
- Presence of active substance-use disorder or concern for dextromethorphan misuse/diversion
Pharmacodynamics and mechanism of action
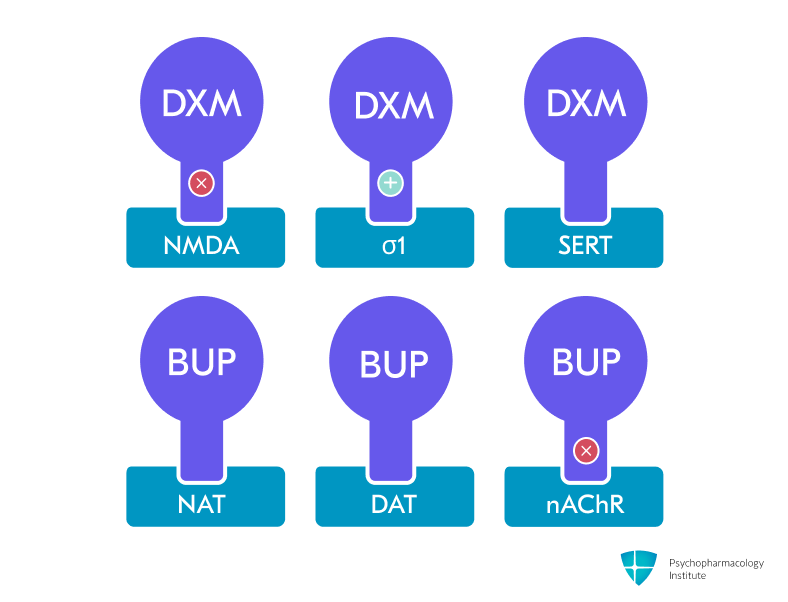
- Dextromethorphan (DXM) component:
- Uncompetitive NMDA-receptor antagonist and high-affinity σ-1 receptor agonist [3]
- Additional actions: SERT/NET inhibition, nicotinic α4β2 antagonism, weak μ-opioid agonism. [4]
- NMDA receptor antagonism (dextromethorphan component)
- Provides glutamatergic modulation similar to ketamine and esketamine [4]
- May contribute to faster onset of antidepressant effects [4,5]
- NMDA blockade indirectly enhances AMPA receptor signaling through increased glutamate release and induces downstream cascades involved in neural plasticity [4,6]
- Increases BDNF release, potentially explaining rapid synaptogenic and antidepressant effects [6,7]
- Activates mTOR signaling pathway, leading to increased protein synthesis and dendritic spine formation in prefrontal cortex [8]
- σ-1 receptor agonism (dextromethorphan component)
- May contribute to antidepressant effects through modulation of neuroplasticity and neuroprotection [6,9,10]
- Bupropion component:
- Norepinephrine- and dopamine-reuptake inhibitor (NDRI) and potent competitive CYP2D6 inhibitor
- CYP2D6 blockade raises dextromethorphan (DXM) exposure and extends its half-life approximately 3-fold to 22 hours [3]
Pharmacokinetics
Metabolism and Pharmacokinetic Interactions
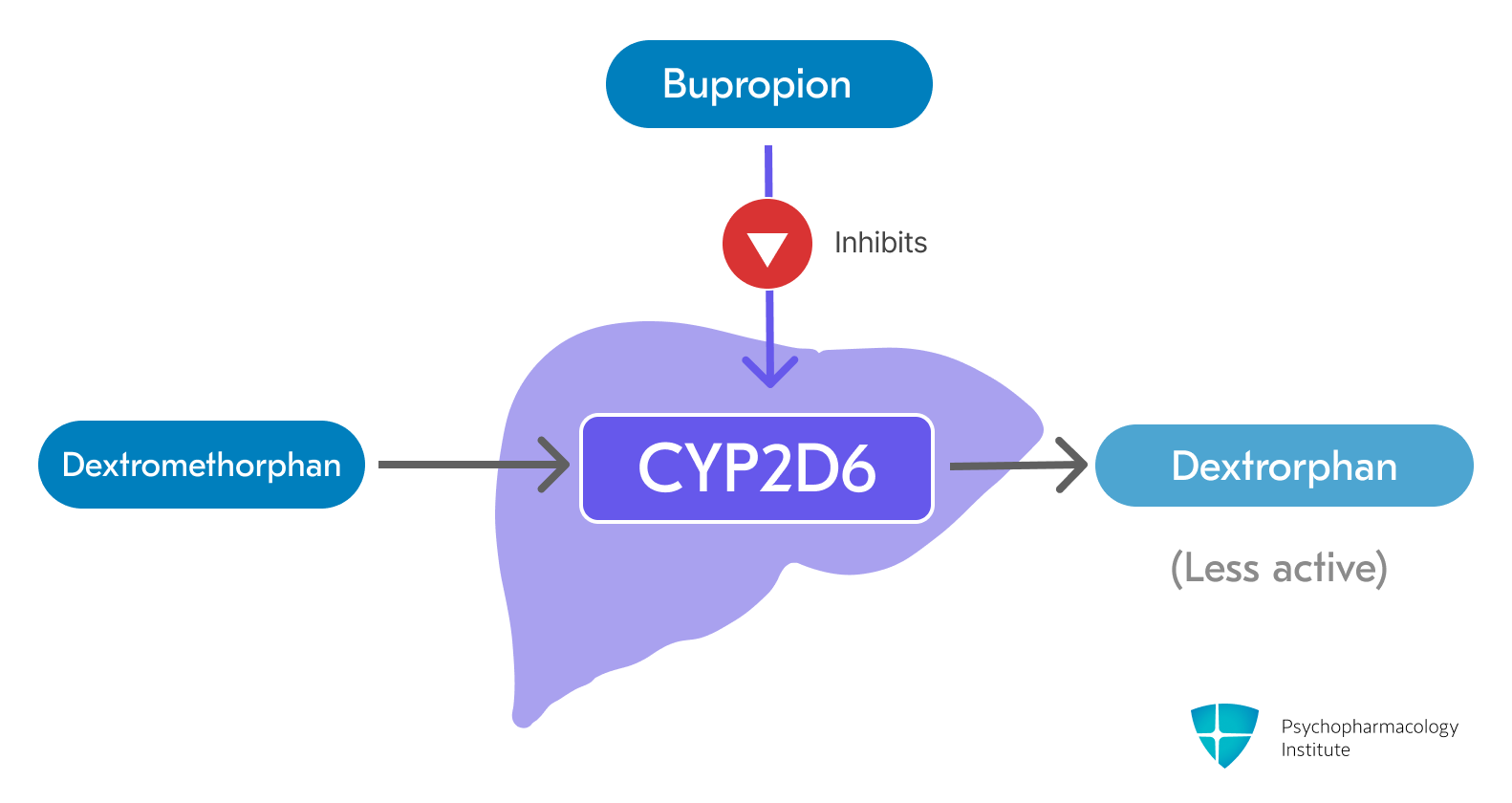
- Dextromethorphan is primarily metabolized by the CYP2D6 enzyme to its major, less active metabolite, dextrorphan [3]
-
Bupropion and its metabolites are strong, competitive inhibitors of CYP2D6 [3]
- This inhibition significantly increases the plasma concentration and extends the half-life of dextromethorphan
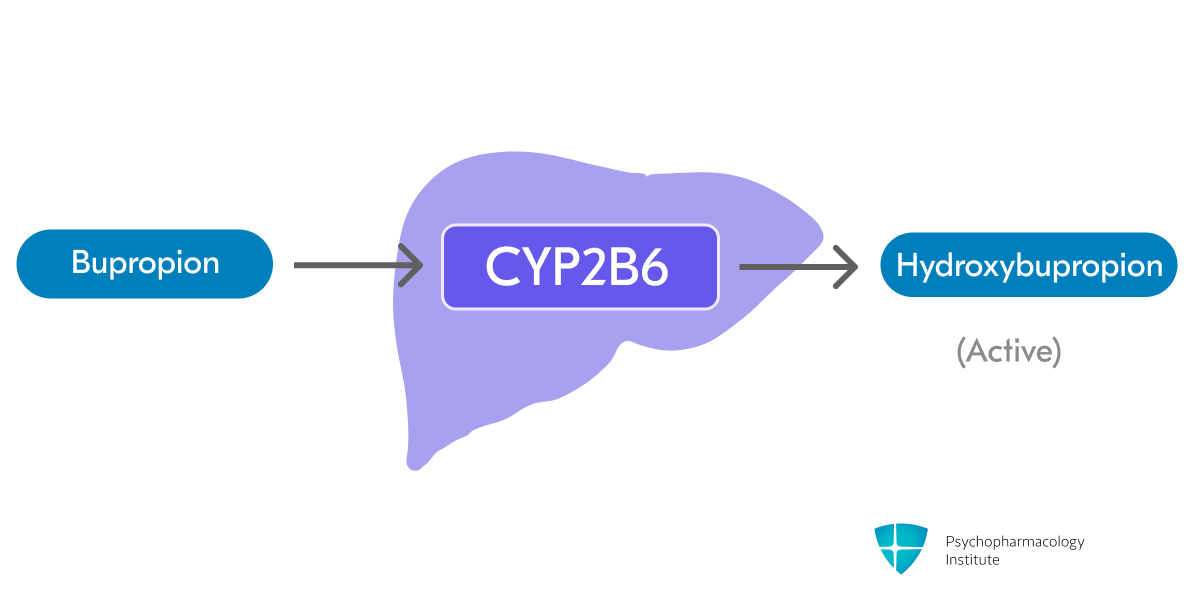
- Bupropion is metabolized in the liver through CYP2B6 to form active metabolite hydroxybupropion (primary pathway) [3,11]
- Threohydrobupropion and erythrohydrobupropion are formed through non-CYP-mediated metabolism (secondary pathway)
-
Dextromethorphan/Bupropion levels increased by:
- Strong CYP2D6 inhibitors (e.g., paroxetine, fluoxetine, quinidine)
- Further increase dextromethorphan concentrations beyond the effect of bupropion alone
- Reduce Dextromethorphan/Bupropion dose to one tablet once daily in the morning [3]
- CYP2B6 Inhibitors (e.g., clopidogrel)
- May increase plasma concentrations of both bupropion and dextromethorphan.
- Monitor for adverse effects [3]
- Strong CYP2D6 inhibitors (e.g., paroxetine, fluoxetine, quinidine)
-
Dextromethorphan/Bupropion levels decreased by:
- Strong CYP2B6 inducers (e.g., carbamazepine, rifampin, phenytoin, efavirenz)
- Significantly decrease plasma concentrations of both bupropion and dextromethorphan, which may reduce efficacy
- Co-administration should be avoided [3]
- Strong CYP2B6 inducers (e.g., carbamazepine, rifampin, phenytoin, efavirenz)
-
Bupropion and its metabolites may increase levels of CYP2D6 substrates, including:
- Antidepressants (venlafaxine, duloxetine, nortriptyline, imipramine, desipramine, paroxetine, fluoxetine, sertraline)
- Vortioxetine dose should be reduced by 50% when used with bupropion. Consider alternative options.
- Antipsychotics (haloperidol, risperidone, aripiprazole)
- Combined use with iloperidone lowers seizure threshold and augments QT-prolongation risk. Consider alternative options.
- Beta-blockers (metoprolol)
- Type 1C antiarrhythmics (propafenone, flecainide)
- Consider dose reduction of CYP2D6 substrates when used concomitantly
- Conversely, drugs that require CYP2D6 for activation (e.g., tamoxifen) may have reduced efficacy
- Antidepressants (venlafaxine, duloxetine, nortriptyline, imipramine, desipramine, paroxetine, fluoxetine, sertraline)
Pharmacodynamic Interactions
- MAOIs
- Contraindicated due to high risk of hypertensive crisis and serotonin syndrome
- A 14-day washout period is required when switching to or from an MAOI [3]
- Serotonergic drugs (e.g., SSRIs, SNRIs, TCAs, triptans)
- Concomitant use increases the risk of serotonin syndrome
- Monitor closely for symptoms. If serotonin syndrome occurs, discontinue [3]
- Drugs that lower seizure threshold (e.g., other antidepressants, antipsychotics, theophylline, systemic corticosteroids)
- Additive risk of seizures due to the bupropion component
- Use with extreme caution. If a seizure occurs, Dextromethorphan/Bupropion must be permanently discontinued [3]
- Dopaminergic drugs (e.g., levodopa, amantadine)
- May increase the risk of CNS toxicity (restlessness, agitation, tremor)
- Use with caution and monitor for adverse effects [3]
- Digoxin
- Bupropion may decrease plasma digoxin levels.
- Monitor digoxin levels upon initiation of co-administration [3]
- Alcohol
- May increase the risk of neuropsychiatric adverse events or reduce alcohol tolerance
- Alcohol consumption should be minimized or avoided [3]
- False-positive drug screens
- Bupropion can cause false-positive urine immunoassay tests for amphetamines [12,13]
- Confirmatory tests (e.g., GC/MS) will distinguish bupropion from amphetamines
Half-life
- After reaching steady state (within 8 days), the mean elimination half-life is:
- Dextromethorphan: ~22 hours [3]
- Bupropion: ~15 hours [3]
- Bupropion’s active metabolites have longer half-lives (threohydrobupropion: ~33 hours; erythrohydrobupropion: ~44 hours)
Dosage forms
- Extended-release:
- Tablets
- 45 mg dextromethorphan hydrobromide/105 mg bupropion hydrochloride
- Auvelity
- Tablets
- Generic substitution considerations:
- Dextromethorphan:
- Available as prescription or over-the-counter generic syrup (7.5 mL typically equals 45 mg dose)
- Significantly lower cost (~$20/month vs $1,200/month for branded combination)
- Bupropion:
- Generic immediate-release: 100 mg twice daily or 200 mg once daily provides similar dosing to the 105 mg component in branded combination
- Dextromethorphan:
- Formulation considerations:
- May be taken with or without meals
- Tablets must be swallowed whole
- Cannot be crushed, divided, or chewed
Indications
FDA-Approved Indications
Major Depressive Disorder (MDD)
- Only oral NMDA receptor antagonist combination approved for adults with MDD [3]
- Rapid onset of action: separation from placebo on MADRS by week 1 and sustained superiority at week 6 in the pivotal GEMINI trial [3,5]
- Superiority over bupropion SR alone at week 6 was reported in the ASCEND study, supporting the specific contribution of dextromethorphan to overall efficacy [14]
- May be considered for patients requiring faster symptom relief or those with inadequate response to first-line antidepressants
- However, limited long-term comparative data, high cost, and dextromethorphan’s diversion potential can temper routine use. [1]
- Durability question: An unpublished TRD trial showed early separation from bupropion at weeks 1–2, but this advantage was not maintained at week 6, highlighting the need for confirmatory results [1,2]
- Dosing:
- Starting dose: One tablet (45 mg dextromethorphan/105 mg bupropion) once daily in the morning [3].
- Target dose: After 3 days, increase to the maximum recommended dosage of one tablet twice daily, with doses separated by at least 8 hours [3]
- Maximum dose: 90 mg dextromethorphan/210 mg bupropion (two tablets per day) [3]
- Strong CYP2D6 inhibitors or known CYP2D6 poor metabolizers: 1 tablet once daily [3]
Off-label Uses
- Treatment-resistant depression and cognitive/anxiety symptoms in MDD are being explored in open-label and extension studies (e.g., COMET-TRD, EVOLVE); evidence is preliminary and not guideline-endorsed. [1]
- There are currently no well-established off-label uses for the dextromethorphan-bupropion combination.
Side effects
Most common side effects
Neurological
- Dizziness (16% incidence)
- Most common side effect [3,5]
- Take precautions to reduce fall risk, particularly in patients with motor impairment or a history of falls
- Caution patients about operating machinery and driving until they know how Auvelity affects them
- Headache (8% incidence) [3,14]
- Somnolence (7% incidence)
- Despite containing bupropion, which is typically activating
- Consider morning dosing if sedation persists
Gastrointestinal
- Nausea (13% incidence)
- Less likely to lead to discontinuation compared to SSRIs [5]
- Can be minimized by taking with food
- Generally improves within the first week of treatment
- Diarrhea (7% incidence)
- Usually mild and self-limiting [14]
- Ensure adequate hydration if persistent
- Dry mouth (6% incidence)
- Sugar-free gum or lozenges may help
- Constipation (4% incidence)
Other common side effects
- Sexual dysfunction (6% incidence)
- Significantly lower than SSRIs/SNRIs
- Bupropion component may actually mitigate sexual side effects [15]
- Consider Dextromethorphan/Bupropion for patients with SSRI-induced sexual dysfunction
- Hyperhidrosis (5% incidence) [3].
- May require dose adjustment if bothersome
- Anxiety (4% incidence)
- Leading adverse reaction causing study discontinuation (2% of patients) in clinical trials [3].
- Monitor during the initial titration period
- Insomnia (4% incidence)
- Ensure doses are separated by at least 8 hours
- Avoid evening doses
Severe side effects
- Seizures
- Dose-related risk inherent to bupropion [3]
- Contraindicated in patients with seizure disorder, eating disorders (especially bulimia), or undergoing abrupt discontinuation of alcohol/benzodiazepines
- Risk factors include: head trauma, CNS tumors, metabolic disorders, concomitant medications lowering seizure threshold [3]
- Do not exceed the maximum dose of 2 tablets daily. Screen patients for use of other bupropion-containing products before initiating treatment.
- Hypertension and cardiovascular effects
- Risk increased with MAOIs, nicotine replacement, or drugs that increase dopaminergic/noradrenergic activity [3]
- Monitor blood pressure before initiation and periodically during treatment
- Use caution in patients with pre-existing hypertension or cardiovascular disease
- Activation of mania/hypomania
- As with other antidepressants, there is a risk of precipitating a manic or hypomanic episode. Screen for bipolar disorder before initiation [3]
- Case report described dextromethorphan-induced manic symptoms in a bipolar patient on lithium [16]
- Interestingly, the combination of dextromethorphan and memantine was assessed in a clinical trial for treating bipolar disorder [17]
- Monitor for the emergence of manic symptoms, especially in the first few weeks
- Serotonin syndrome
- Risk with concomitant SSRIs, SNRIs, tricyclics, triptans, or other serotonergic agents [3]
- Contraindicated with MAOIs (14-day washout required) [3]
- Monitor for symptoms: hyperthermia, muscle rigidity, autonomic instability, mental status changes
- Psychosis and neuropsychiatric reactions
- Bupropion can cause delusions, hallucinations, paranoia, confusion [3,18]
- Dextromethorphan overdose can cause toxic psychosis [19]
- Risk increased with higher doses or in predisposed individuals
- Discontinue if psychotic symptoms emerge
- Angle-closure glaucoma
- Pupilary dilation induced by bupropion may trigger angle-closure attack in patients with anatomically narrow angles [3,20]
- Screen patients with narrow angles who have not had iridectomy
- Educate about symptoms: eye pain, vision changes, eye redness/swelling
Abuse potential
- Dextromethorphan is available as an over-the-counter antitussive that produces intoxicating, hallucinogenic, and dissociative effects at supra-therapeutic doses [21]
- Recreational use of DXM is sometimes referred to in slang form as “robo-tripping” or “skittling”, whose prefix derives from the Robitussin brand name, or “Triple Cs” [22]
- Fatal overdoses have been reported in cases where death was attributed to dextromethorphan toxicity [23]
- Known as “poor man’s cocaine” due to its stimulant-like effects, bupropion has earned this street name and is the most commonly misused antidepressant, with 75% increase in abuse between 2000-2012 [24–26]
- Monitor for signs of misuse, especially in patients with a substance use history
Use in special populations
Pregnancy
- Not recommended: Animal studies show fetal harm and neurotoxicity concerns.
- Manufacturer recommends discontinuing treatment in pregnant females [3]
- Use alternative treatment for women who are planning to become pregnant.
Breastfeeding
- Breast-feeding is not recommended during therapy and for 5 days after the final dose because of potential neurotoxicity [3]
- Bupropion and active metabolites appear in human milk (≈ 2% of the weight-adjusted maternal dose).
- Dextromethorphan levels in human milk are unknown.
Hepatic Impairment
- Mild to moderate impairment (Child-Pugh A or B):
- No dose adjustment needed [3]
- Severe hepatic impairment (Child-Pugh C):
- Use is not recommended (has not been studied) [3]
Renal Impairment
- Mild impairment (eGFR ≥60 mL/minute/1.73 m²):
- No dosage adjustment necessary
- Moderate impairment (eGFR 30-59 mL/minute/1.73 m²):
- Reduce to one tablet once daily in the morning [3]
- Severe impairment/End-stage renal disease (eGFR <30 mL/minute/1.73 m²):
- Use is not recommended (has not been studied) [3]
CYP2D6 Poor Metabolizers
- Dosage adjustment required: One tablet once daily in the morning [3]
- Poor metabolizers have approximately 3-fold higher dextromethorphan concentrations.
Elderly
- No patients ≥ 65 years were enrolled in pivotal trials; pharmacokinetics unstudied.
Brand names
- US: Auvelity
References
- McCarthy, B., Bunn, H., Santalucia, M., Wilmouth, C., Muzyk, A., & Smith, C. M. (2023). Dextromethorphan-bupropion (Auvelity) for the Treatment of Major Depressive Disorder. Clinical Psychopharmacology and Neuroscience, 21(4), 609–616. https://doi.org/10.9758/cpn.23.1081
- Read, 18. M. (2020, March 30). Axsome Therapeutics Announces Topline Results of the STRIDE-1 Phase 3 Trial in Treatment Resistant Depression and Expert Call to Discuss Clinical Implications. BioSpace. https://www.biospace.com/axsome-therapeutics-announces-topline-results-of-the-stride-1-phase-3-trial-in-treatment-resistant-depression-and-expert-call-to-discuss-clinical-implications
- Food, U. S., & Administration, D. (2024). AUVELITY® (dextromethorphan hydrobromide and bupropion hydrochloride) extended-release tablets, for oral use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/215430s008lbl.pdf
- Stahl, S. M. (2019). Dextromethorphan/Bupropion: A Novel Oral NMDA (N-methyl-d-aspartate) Receptor Antagonist with Multimodal Activity. CNS Spectrums, 24(5), 461–466. https://doi.org/10.1017/S1092852919001470
- Iosifescu, D. V., Jones, A., O’Gorman, C., Streicher, C., Feliz, S., Fava, M., & Tabuteau, H. (2022). Efficacy and Safety of AXS-05 (Dextromethorphan-Bupropion) in Patients With Major Depressive Disorder: A Phase 3 Randomized Clinical Trial (GEMINI). The Journal of Clinical Psychiatry, 83(4), 21m14345. https://doi.org/10.4088/JCP.21m14345
- Zanos, P., Moaddel, R., Morris, P. J., Riggs, L. M., Highland, J. N., Georgiou, P., Pereira, E. F. R., Albuquerque, E. X., Thomas, C. J., Zarate, C. A., & Gould, T. D. (2018). Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacological Reviews, 70(3), 621–660. https://doi.org/10.1124/pr.117.015198
- McIntyre, R. S., Rosenblat, J. D., Nemeroff, C. B., Sanacora, G., Murrough, J. W., Berk, M., Brietzke, E., Dodd, S., Gorwood, P., Ho, R., Iosifescu, D. V., Lopez Jaramillo, C., Kasper, S., Kratiuk, K., Lee, J. G., Lee, Y., Lui, L. M. W., Mansur, R. B., Papakostas, G. I., … Stahl, S. (2021). Synthesizing the Evidence for Ketamine and Esketamine in Treatment-Resistant Depression: An International Expert Opinion on the Available Evidence and Implementation. American Journal of Psychiatry, 178(5), 383–399. https://doi.org/10.1176/appi.ajp.2020.20081251
- Duman, R. S., Aghajanian, G. K., Sanacora, G., & Krystal, J. H. (2016). Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nature Medicine, 22(3), 238–249. https://doi.org/10.1038/nm.4050
- Hashimoto, K. (2009). Sigma-1 receptors and selective serotonin reuptake inhibitors: Clinical implications of their relationship. Central Nervous System Agents in Medicinal Chemistry, 9(3), 197–204. https://doi.org/10.2174/1871524910909030197
- Fishback, J. A., Robson, M. J., Xu, Y.-T., & Matsumoto, R. R. (2010). Sigma receptors: Potential targets for a new class of antidepressant drug. Pharmacology & Therapeutics, 127(3), 271–282. https://doi.org/10.1016/j.pharmthera.2010.04.003
- Costa, R., Oliveira, N. G., & Dinis-Oliveira, R. J. (2019). Pharmacokinetic and pharmacodynamic of bupropion: Integrative overview of relevant clinical and forensic aspects. Drug Metabolism Reviews, 51(3), 293–313. https://doi.org/10.1080/03602532.2019.1620763
- Casey, E. R., Scott, M. G., Tang, S., & Mullins, M. E. (2011). Frequency of False Positive Amphetamine Screens due to Bupropion Using the Syva Emit II Immunoassay. Journal of Medical Toxicology, 7(2), 105–108. https://doi.org/10.1007/s13181-010-0131-5
- Battini, V., Cirnigliaro, G., Giacovelli, L., Boscacci, M., Manzo, S. M., Mosini, G., Guarnieri, G., Gringeri, M., Benatti, B., Clementi, E., Dell’Osso, B., & Carnovale, C. (2023). Psychiatric and non-psychiatric drugs causing false-positive amphetamines urine test in psychiatric patients: A pharmacovigilance analysis using FAERS. Expert Review of Clinical Pharmacology. https://www.tandfonline.com/doi/abs/10.1080/17512433.2023.2211261
- Tabuteau, H., Jones, A., Anderson, A., Jacobson, M., & Iosifescu, D. V. (2022). Effect of AXS-05 (Dextromethorphan-Bupropion) in Major Depressive Disorder: A Randomized Double-Blind Controlled Trial. The American Journal of Psychiatry, 179(7), 490–499. https://doi.org/10.1176/appi.ajp.21080800
- Clayton, A. H., Warnock, J. K., Kornstein, S. G., Pinkerton, R., Sheldon-Keller, A., & McGarvey, E. L. (2004). A placebo-controlled trial of bupropion SR as an antidote for selective serotonin reuptake inhibitor-induced sexual dysfunction. The Journal of Clinical Psychiatry, 65(1), 62–67. https://doi.org/10.4088/jcp.v65n0110
- Bostwick, J. M. (1996). Dextromethorphan-induced manic symptoms in a bipolar patient on lithium. Psychosomatics, 37(6), 571–573. https://doi.org/10.1016/S0033-3182(96)71523-2
- Lee, S.-Y., Wang, T.-Y., Chen, S.-L., Chang, Y.-H., Chen, P.-S., Huang, S.-Y., Tzeng, N.-S., Wang, L.-J., Lee, I.-H., Chen, K.-C., Yang, Y.-K., Hong, J.-S., & Lu, R.-B. (2020). Combination of dextromethorphan and memantine in treating bipolar spectrum disorder: A 12-week double-blind randomized clinical trial. International Journal of Bipolar Disorders, 8, 11. https://doi.org/10.1186/s40345-019-0174-8
- Omri, M., Ferhi, M., Rauschenbach, C., Ibrahim, A., Oliveira Galvao, M., & Hamm, O. (2024). Understanding De Novo Bupropion-Induced Psychosis and Its Management Strategies: A Case Report and Literature Review. Cureus. https://doi.org/10.7759/cureus.73980
- Price, L. H., & Lebel, J. (2000). Dextromethorphan-Induced Psychosis. American Journal of Psychiatry, 157(2), 304–304. https://doi.org/10.1176/appi.ajp.157.2.304
- Narayanan, V. (2019). Ocular Adverse Effects of Antidepressants – Need for an Ophthalmic Screening and Follow up Protocol. Ophthalmology Research: An International Journal, 1–6. https://doi.org/10.9734/or/2019/v10i330107
- Gupta, L., Tomar, N., & Sarin, R. K. (2024). Dextromethorphan: A double-edged drug – Unveiling the pernicious repercussions of Abuse and forensic implications. Emerging Trends in Drugs, Addictions, and Health, 4, 100161. https://doi.org/10.1016/j.etdah.2024.100161
- Stanciu, C. N., Penders, T. M., & Rouse, E. M. (2016). Recreational use of dextromethorphan, “Robotripping”-A brief review. The American Journal on Addictions, 25(5), 374–377. https://doi.org/10.1111/ajad.12389
- Logan, B. K., Goldfogel, G., Hamilton, R., & Kuhlman, J. (2009). Five deaths resulting from abuse of dextromethorphan sold over the internet. Journal of Analytical Toxicology, 33(2), 99–103. https://doi.org/10.1093/jat/33.2.99
- Evans, E. A., & Sullivan, M. A. (2014). Abuse and misuse of antidepressants. Substance Abuse and Rehabilitation, 5, 107–120. https://doi.org/10.2147/SAR.S37917
- Stassinos, G. L., & Klein-Schwartz, W. (2016). Bupropion “abuse” reported to us poison centers. Journal of Addiction Medicine, 10(5), 357–362. https://doi.org/10.1097/ADM.0000000000000249
- Kaur, J., Modesto-Lowe, V., & León-Barriera, R. (2024). Do not overlook bupropion misuse. Primary Care Companion for CNS Disorders, 26(2), 54349. https://doi.org/10.4088/PCC.23lr03685



