In a Nutshell
- New molecular entities (NMEs):
- Cobenfy (xanomeline-trospium) targeting schizophrenia through M1/M4 agonism.
- Kisunla (donanemab-azbt) for mild Alzheimer’s with confirmed amyloid pathology.
- Expanded indications:
- Fanapt (iloperidone) for the treatment of manic or mixed episodes in bipolar I disorder.
- New formulations:
- Ingrezza Sprinkle (valbenazine) for patients with tardive dyskinesia and dysphagia.
- Onyda XR (clonidine) as a liquid suspension for pediatric ADHD.
- Erzofri (paliperidone palmitate) with a simplified single first-month injection.
- New prodrug:
- Zunveyl (benzgalantamine), designed to reduce GI side effects in Alzheimer’s disease.
ADHD
Onyda XR (Clonidine) – May 30, 2024
- Onyda XR is a new liquid formulation of clonidine, an alpha2-adrenergic agonist already approved for ADHD.
- Indication:
- Treatment of ADHD in pediatric patients aged 6 years and older [1].
- The manufacturer used a proprietary technology to transform the existing formulation into an extended-release liquid suspension.
- The FDA approved Onyda XR under the 505(b)(2) regulatory pathway, which allows the use of existing clinical data from previously approved drugs [2] , [3] .
- According to the manufacturer, Onyda XR offers two potential benefits [4]:
- It may be easier for children with difficulty swallowing pills as an orange-flavored liquid formulation.
- Being a once-daily medication taken at night, it could help simplify morning routines.
Bipolar Disorder
Fanapt (Iloperidone) – April 3, 2024
- Iloperidone (Fanapt) is now approved for the treatment of manic or mixed episodes associated with bipolar I disorder [5].
- This approval represents an expanded indication, as iloperidone was already approved for the treatment of schizophrenia [6].
- Iloperidone requires a 7-day titration period to minimize the risk of orthostatic hypotension.
Alzheimer’s Disease
Zunveyl (Benzgalantamine) – July 29, 2024
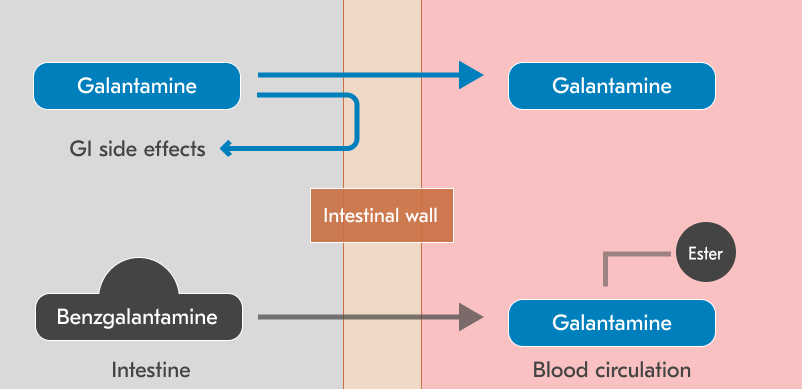
- Zunveyl (Benzgalantamine) is a benzoyl ester prodrug of galantamine.
- Indication:
- Mild-to-moderate dementia in patients with Alzheimer’s disease [7].
- Pharmacology:
- Galantamine therapy is often limited by gastrointestinal side effects [8], due to cholinergic stimulation of the enteric nervous system.
- Benzgalantamine was designed with a benzoyl ester modification that prevents binding to intestinal cholinergic receptors, reducing GI side effects [9].
- Following absorption, benzgalantamine is hydrolyzed in the bloodstream to release the active galantamine and benzoic acid.
- The prodrug approach aims to maintain therapeutic efficacy while potentially improving tolerability.
Kisunla (Donanemab-Azbt) – July 2, 2024
- Pharmacology
- Donanemab is a monoclonal antibody that reduces the accumulation of amyloid beta plaques.
- Indication [10]:
- Patients with mild cognitive impairment or mild dementia stage of Alzheimer’s disease, who have confirmed amyloid pathology.
- Efficacy:
- Reduces the rate of cognitive and functional decline in mild cognitive impairment and mild dementia stage patients.
- Side effects
- Boxed warning for intracerebral hemorrhage risk, more specifically “amyloid-related imaging abnormalities (ARIA)”.
- Practical aspects [11]:
- The cost is estimated at USD 32,000 for 12 months of treatment and USD 48,696 for 18 months.
- IV infusions are given every 4 weeks.
- Requires MRI monitoring.
- It can be discontinued after the plaque is cleared.
Schizophrenia and Related Disorders
Erzofri (Paliperidone Palmitate) – July 28, 2024
- Erzofri is a new monthly formulation of paliperidone palmitate, comparable to Invega Sustenna [12] .
- Key difference:
- Erzofri requires a single injection during the first month, while Invega Sustenna requires two injections [13].
- Indications :
- Schizophrenia in adults.
- Schizoaffective disorder in adults, as a monotherapy or adjunct to mood stabilizers or antidepressants.
Ingrezza Sprinkle (Valbenazine) – April 30, 2024
- Ingrezza Sprinkle is a new valbenazine formulation approved for treating tardive dyskinesia and chorea associated with Huntington’s disease [14].
- The new sprinkle formulation is aimed mostly at patients with dysphagia.
- The sprinkle capsule is designed to be opened and sprinkled over a small amount of soft food, such as applesauce, yogurt, or pudding. It should not be mixed into liquids like milk or water.
Cobenfy (Xanomeline-Trospium) – September 26, 2024
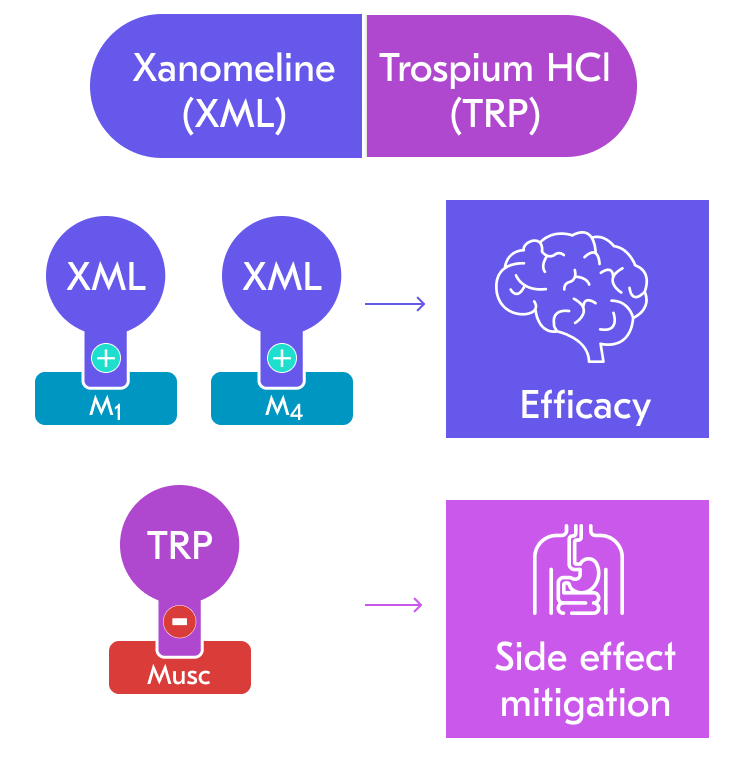
- Pharmacology:
- Cobenfy combines xanomeline for therapeutic effects and trospium chloride to reduce cholinergic side effects.
- Xanomeline, an M1 and M4 agonist, modulates dopamine release in psychosis-related areas [15].
- M1 agonism enhances prefrontal cortex GABAergic inhibition, reducing glutamate signaling and VTA dopamine release.
- M4 agonism activates cholinergic autoreceptors, decreasing acetylcholine and subsequent VTA dopamine release.
- Both mechanisms target psychosis-related striatal areas, potentially offering antipsychotic effects with fewer motor side effects.
- Trospium is a peripheral muscarinic antagonist that prevents excess cholinergic effects.
- Indication:
- Treatment of schizophrenia in adults
- Common side effects (≥5% and at least twice placebo) [16]
- Nausea, dyspepsia, constipation, vomiting, hypertension, abdominal pain, diarrhea, tachycardia, dizziness, and GERD.
- Contraindications:
- Urinary retention, moderate or severe hepatic impairment, gastric retention, and untreated narrow-angle glaucoma.
- Estimated cost: USD 1850/month
References
- U.S. Food and Drug Administration. (2024). ONYDA XR (clonidine hydrochloride) extended-release oral suspension: Prescribing Information (Drug Label 217645). Center for Drug Evaluation and Research. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/217645s000lbl.pdf
- U.S. Food and Drug Administration. (2024). NDA 217645 approval package for: ONYDA XR (clonidine hydrochloride) (New Drug Application Approval 217645Orig1s000). Center for Drug Evaluation and Research. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2024/217645Orig1s000Approv.pdf
- Goldstein, B. (2023). Overview of the 505(b)(2) regulatory pathway for new drug applications [Regulatory Guidance Presentation]. U.S. Food and Drug Administration / Center for Drug Evaluation and Research. https://www.fda.gov/media/156350/download
- Tris Pharma, Inc. (n.d.). ONYDA™ XR (clonidine HCI) ADHD medication. https://onyda.com
- U.S. Food and Drug Administration. (2024). Supplemental new drug application approval for FANAPT (iloperidone) tablets (FDA Approval Letter NDA 022192/S-023). U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2024/022192Orig1s023ltr.pdf
- Vanda Pharmaceuticals Inc. (2024). FANAPT (iloperidone) tablets: Prescribing information. Vanda Pharmaceuticals Inc. https://www.fanaptpro.com/wp-content/uploads/Fanapt-Prescribing-Information.pdf
- Alpha Cognition Inc. (2024). ZUNVEYL (benzgalantamine) delayed-release tablets, for oral use: Highlights of prescribing information. Alpha Cognition Inc. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/218549s000lbl.pdf
- Sridhar, G. R. (2022). Acetylcholinesterase inhibitors (Galantamine, Rivastigmine, and Donepezil). In P. Riederer, G. Laux, T. Nagatsu, W. Le, & C. Riederer (Eds.), NeuroPsychopharmacotherapy (pp. 2709–2721). Springer International Publishing. https://doi.org/10.1007/978-3-030-62059-2_418
- Alpha Cognition Inc. (2024). A new approach for the treatment and administration of Alzheimer’s disease. https://www.alphacognition.com/treatments/alzheimers-alpha-1062/
- U.S. Food and Drug Administration. (2024). KISUNLA (donanemab-azbt) injection: Prescribing information (New Drug Application Approval NDA 218390). U.S. Food and Drug Administration / U.S. Food and Drug Administration. https://www.fda.gov/media/180803/download
- Richmond, L. M. (2024). New medication, staging criteria signal a potential shift in alzheimer’s care. Psychiatric News, 59(8). https://doi.org/10.1176/appi.pn.2024.08.8.46
- Shandong Luye Pharmaceutical Co., Ltd. (2024). ERZOFRI (paliperidone palmitate) prescribing information. https://dailymed.nlm.nih.gov/dailymed/getFile.cfm?setid=492bf9dd-868e-421a-92db-8cca8973aac1&type=pdf
- Janssen Pharmaceuticals, Inc. (2024). INVEGA SUSTENNA (paliperidone palmitate) prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2024/022264s037lbledt.pdf
- U.S. Food and Drug Administration. (2024). INGREZZA SPRINKLE (valbenazine) capsules: FDA Approval (New Drug Application Approval NDA 218390). U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2024/218390Orig1s000ltr.pdf
- Paul, S. M., Yohn, S. E., Brannan, S. K., Neugebauer, N. M., & Breier, A. (2024). Muscarinic Receptor Activators as Novel Treatments for Schizophrenia. Biological Psychiatry, 96(8), 627–637. https://doi.org/10.1016/j.biopsych.2024.03.014
- Bristol-Myers Squibb. (2024). COBENFY (xanomeline and trospium chloride) [package insert]. Princeton, NJ: Author. https://packageinserts.bms.com/pi/pi_cobenfy.pdf



