In a nutshell
This year’s major developments center on improved access, refined labeling, and updated clinical guidance. Esketamine gained monotherapy approval for TRD. Lumateperone joined the adjunctive options for MDD. New APA guidance recommends against antipsychotics for delirium prevention. And the first joint guideline on benzodiazepine tapering emphasizes gradual, hyperbolic reductions.

What Changed in 2025 (High-Yield Updates)
- Delirium: The new APA guidance recommends against antipsychotics for prevention or routine treatment; reserve for severe distress/unsafe behavior after nonpharmacologic strategies and reversible contributors are addressed
- Benzodiazepines: Joint tapering guidance emphasizes individualized, gradual tapers; avoid abrupt discontinuation; consider “hyperbolic” reductions as doses get lower.
- TRD: Esketamine (Spravato) gained TRD monotherapy labeling; primarily a labeling-flexibility change rather than a new “superior efficacy” claim (REMS, clinic workflow, and access barriers still shape real-world use).
- MDD: Lumateperone (Caplyta) joined the FDA-approved adjunct options for MDD, adding another atypical antipsychotic alternative alongside established adjuncts.
- Bipolar maintenance: Risperidone extended-release injectable suspension for subcutaneous use (UZEDY) was approved as monotherapy or as adjunctive therapy to lithium or valproate for the maintenance treatment of bipolar I disorder in adults.
- For bipolar maintenance, use the monthly regimen (the q2-month schedule is not recommended per labeling).
- Schizophrenia: A historic reduction in administrative burden.
- Pharmacies no longer need REMS enrollment to dispense; prescribers do not need to register patients in a federal database.
- Prescribers must still monitor ANC regarding the boxed warning for neutropenia.
- Addiction: Buprenorphine Extended-Release (Sublocade) rapid initiation label update targets a real-world attrition point by allowing transition to injection after minimal transmucosal exposure, rather than requiring a prolonged “tolerance” lead-in.
- Fibromyalgia Cyclobenzaprine sublingual (Tonmya) gained approval in a low-dose sublingual formulation that targets sleep-pain cycle.
- Alzheimer’s Disease:
- Donanemab (Kisunla) slower titration aims to reduce ARIA-E risk while maintaining disease-modifying biomarker effects.
- Lumipulse is the first FDA-cleared blood test to aid AD diagnosis in cognitively impaired adults.
- ADHD: FDA class-wide ER stimulant labeling adds a clearer “limitation of use” signal for children <6 years, reflecting higher exposure and higher rates of adverse events (including clinically significant weight loss).
2025 Clinical Guidelines Updates
Delirium: APA Practice Guideline (September 2025)
- The APA’s delirium guideline is the first comprehensive update in over 20 years [1,2]
- Key shift: The guideline recommends against using antipsychotics for delirium prevention or to hasten resolution, reserving them for severe neuropsychiatric symptoms when safety/distress thresholds are met.
- Key highlights:[2]
- Restrictive use of antipsychotics:
- Antipsychotics should not be used to prevent delirium or to hasten delirium resolution.
- Antipsychotics should only be used to address neuropsychiatric disturbances if all the following criteria are met:
- Verbal and nonverbal de-escalation strategies have failed.
- Contributing factors (e.g., pain, infection) have been assessed and addressed.
- The symptoms cause significant distress to the patient or present a risk of physical harm to the patient or others.
- Avoidance of benzodiazepines and certain “sleep aids”:
- Benzodiazepines should not be used in delirium (or those at risk) unless there is a specific indication (e.g., withdrawal syndromes excluded from the guideline’s scope).
- Melatonin and ramelteon should not be used to prevent or treat delirium.
- Preference for dexmedetomidine in critical care:
- For patients undergoing major surgery or receiving mechanical ventilation in a critical care setting, the APA suggests using dexmedetomidine rather than other sedating agents to prevent delirium.
- Restrictive use of antipsychotics:
Benzodiazepine Tapering: Joint Clinical Practice Guideline
- Joint Clinical Practice Guideline developed through systematic review and clinical consensus by a Clinical Guideline Committee (CGC) from the American Society of Addiction Medicine and endorsed by several other societies [3]
- Presents recommendations for clinicians across various settings on how to manage and taper benzodiazepine (BZD) use.
- Highlights and Core Tapering Principles[3]
- Physical dependence vs. SUD:
- Physical dependence is an expected biological outcome of regular BZD use and is distinct from Benzodiazepine Use Disorder (SUD)
- When to taper
- Tapering is indicated when a patient is likely physically dependent, and risks now outweigh benefits (e.g., falls, cognitive impairment, misuse, co-prescribed opioids).
- Avoid abrupt discontinuation in any patient likely to be dependent because of withdrawal and seizure risk.
- Tapering strategies and adjustments
- Initial dose reductions should generally be 5% to 10% every 2–4 weeks.
- The taper pace should typically not exceed 25% every 2 weeks.
- Consider hyperbolic tapering (smaller absolute dose reductions over time; e.g., 10 mg → 9 mg → 8.1 mg) to better match changing receptor occupancy at lower doses.
- Hyperbolic tapering is a strategy of nonlinear, sequential dose reductions, meaning the size of the reductions decreases over time.
- Patients who have been taking lower doses for a relatively short period of time (eg, <3 months) may be able to taper more quickly.
- Long-acting agents:
- Consider switching to a longer-acting BZD (e.g., diazepam, clonazepam) before tapering if no contraindications (e.g., severe liver dysfunction).
- Management of co-occurring psychiatric conditions
- Strongly consider tapering benzodiazepines in patients with PTSD, as benzodiazepines are ineffective for core PTSD symptoms and may increase the risk of depression and aggression.
- Physical dependence vs. SUD:
Depression and Mood Disorders
Esketamine (Spravato) – Monotherapy Approval (January 21, 2025)
- Esketamine (Spravato) CIII nasal spray received FDA approval as a monotherapy for adults with Treatment-Resistant Depression (TRD)
- This represents a supplemental New Drug Application (sNDA) approval
- Previously, esketamine was only approved for use in conjunction with an oral antidepressant
- The separate suicidality-related indication still requires use in conjunction with an oral antidepressant [4]
- Indication: Treatment-Resistant Depression (TRD) in adults, now as a standalone therapy (see our Esketamine Brain Guide):
- Monotherapy approval allows clinicians to initiate esketamine without adding a new daily oral antidepressant, which is particularly relevant for patients with:
- Significant intolerance to oral antidepressants (e.g., sexual dysfunction, weight gain, GI side effects).
- High pill burden or polypharmacy, where another oral agent is undesirable.
- Monotherapy approval allows clinicians to initiate esketamine without adding a new daily oral antidepressant, which is particularly relevant for patients with:
- Pivotal Postmarketing Randomized Trial (Study 3; NCT04599855) [4,5]
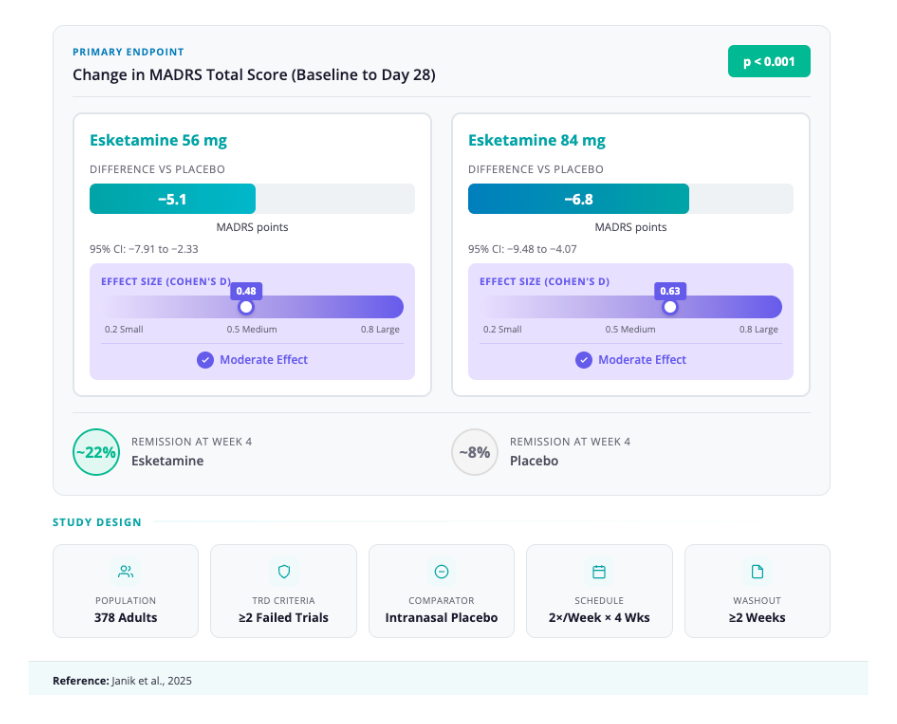
- Design: Multicenter, randomized, double-blind, placebo-controlled; 378 adults with TRD randomized 1:1:2 to SPRAVATO 56 mg, 84 mg, or placebo nasal spray for 4 weeks (twice weekly).
- Primary outcome (Day 28 MADRS): LS mean difference vs placebo −5.1 (56 mg) and −6.8 (84 mg), both P < .001;
- Effect sizes: 0.48 and 0.63, respectively.
- Secondary efficacy end point (Day 2 MADRS, ~24h): Between-group differences significant for both esketamine doses −3.8 (P=.004) for 56 mg and −3.4 (P=.006) for 84 mg.
- Practical Aspects:
- Dosing:
- The schedule remains unchanged from the adjunctive indication (Twice weekly induction, tapering to once weekly/bi-weekly maintenance) [4,5]
- REMS-restricted distribution with in-clinic administration, 2-hour monitoring, and restrictions on driving until the next day.
- Adverse effects remain similar to adjunctive use: dissociation, dizziness, nausea, transient blood pressure increases, and sedation [5,6]
- Dosing:
- Clinical impact & positioning:
- Observed effect sizes were modest–moderate (0.48–0.63). Inferences about how this compares with adjunctive esketamine or other TRD interventions should be made cautiously because populations, designs, endpoints, and expectancy effects differ across trials [7–11]
- As with other rapidly psychoactive interventions, functional unblinding is a potential limitation (participants and raters may infer assignment based on acute subjective effects); overall, interpret cross-trial comparisons cautiously [12]
- In routine practice, REMS-required in-clinic dosing and monitoring, scheduling, transportation, and reimbursement remain key barriers; these factors often drive real-world comparisons with IV ketamine as much as efficacy does.
- The monotherapy approval primarily expands labeling flexibility by allowing esketamine for TRD without mandatory combination with an oral antidepressant
Lumateperone (Caplyta) – Adjunctive MDD (November 6, 2025)
- Lumateperone (Caplyta) received FDA approval as an adjunctive therapy to oral antidepressants for major depressive disorder (MDD) in adults with an inadequate response to standard antidepressant therapy [13]
- Previous indication for lumateperone, already FDA- approved: [14]
- Schizophrenia (adults)
- Treatment of depressive episodes associated with bipolar I or II disorder (bipolar depression) in adults, as monotherapy and as adjunctive therapy with lithium or valproate.
- New indication: Adjunctive treatment of Major Depressive Disorder (MDD) in adults
- Other FDA-approved antipsychotic adjuncts for MDD include [15–17]
- Aripiprazole,
- Brexpiprazole,
- Cariprazine
- Quetiapine XR and
- Olanzapine/fluoxetine combination
- Efficacy Data: Approval was based on two Phase 3 studies (Study 501 and Study 502) [13,18]
- Design: Randomized, double-blind, placebo-controlled global trials
- Population: Adults with MDD and inadequate response to antidepressant therapy
- Intervention: Lumateperone 42 mg + Antidepressant vs. Placebo + Antidepressant
- Duration: 6 weeks
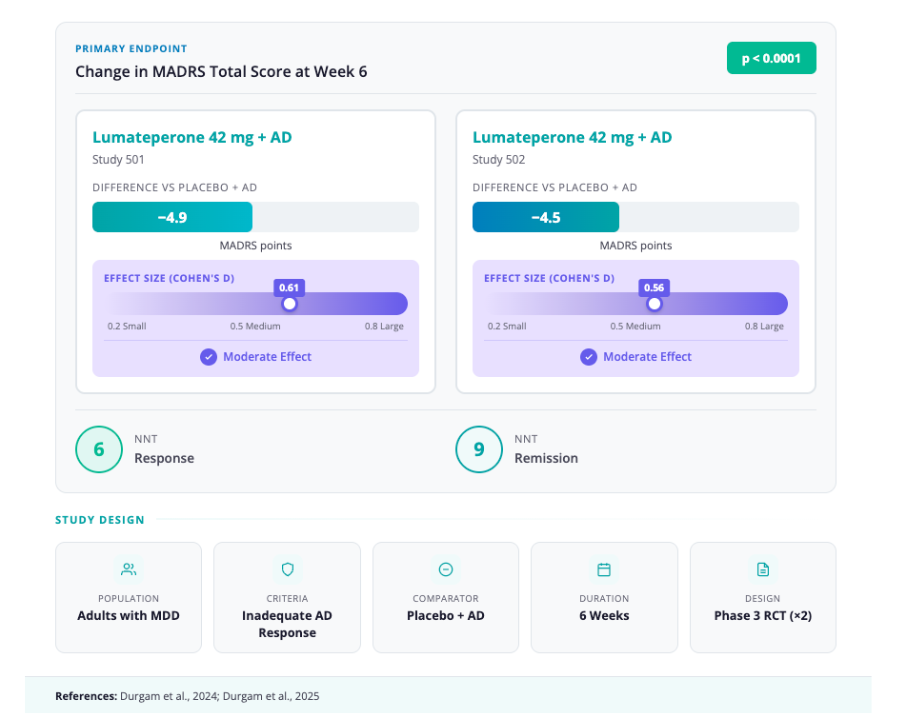
- Primary Outcome: Change in MADRS total score at Week 6
- Key Results:
- Study 501: -4.9 point reduction vs. placebo (effect size 0.61; p < 0.0001) [18]
- Study 502: -4.5 point reduction vs. placebo (effect size 0.56; p < 0.0001)
- Number Needed to Treat (NNT): Approximately 6 for response, 9 for remission [19]
- Side Effects & Safety:
- Metabolic Profile: Lumateperone demonstrates a favorable metabolic profile. In pooled safety data, mean changes in weight, glucose, and lipids were similar to placebo [13,19]
- Common Adverse Events: Dizziness, somnolence, dry mouth, nausea [13,15,19]
- Motor Symptoms: Low risk of extrapyramidal symptoms (EPS) or akathisia, comparable to placebo in trials [13,19]
- Practical Aspects:
- Dosing: One dose (42 mg) for all indications. No titration is required [14]
- Clinical Positioning: Due to its lower metabolic risk compared to agents like olanzapine or quetiapine, it is likely to be favored for patients with pre-existing metabolic concerns or those who have experienced weight gain on prior adjunctive agents [15]
Interventional Psychiatry: Adolescent TMS (August–November 2025)
- 2025 marked a significant expansion for Transcranial Magnetic Stimulation (TMS) in youth.
- Following the clearance of NeuroStar in 2024 for use as an adjunct for the treatment of major depressive disorder (MDD) in adolescent patients aged 15-21 [20], the FDA cleared multiple additional devices for use in adolescents with MDD in 2025.
- Approvals:
- MagVenture (MagPro): Cleared Aug 25, 2025 [21]
- Apollo TMS: Cleared Sept 10, 2025 [22]
- BrainsWay Deep TMS (H-Coil): Cleared Nov 13, 2025 [23]
- BrainsWay submitted RWE from over 1,100 adolescent patients showing a 66.1% response rate [23]
- These approvals were largely driven by the 510(k) regulatory pathway, which allows devices to be cleared by demonstrating “substantial equivalence” to a predicate device (in this case, the initial NeuroStar clearance) or through the submission of Real-World Evidence (RWE) [23]
- Accelerated Protocols:
- BrainsWay also received clearance for an accelerated adult protocol.
- Standard protocol: 1 session/day; 5 days/week for 4 weeks (acute phase) followed by 2 sessions/week for up to 12 weeks (continuation phase) (Preprint available [24])
- Accelerated protocol: 5 sessions/day for 6 days (acute phase), followed by 2 treatment sessions per day, 1 day per week for an additional 4 weeks (continuation phase) (Preprint available [24])
Transcranial Direct Current Stimulation (Flow) – First At-Home Device (December 11, 2025)
- The FDA authorized the first at-home transcranial direct current stimulation (tDCS) headset for the treatment of Major Depressive Disorder (MDD) in adults [25]
- Indication:[25]
- For adults (18+) with moderate-to-severe MDD.
- Approved as both a monotherapy and an adjunctive treatment (used alongside medication)
- In patients not considered treatment refractory to medication.
- Mechanism:
- Utilizes Transcranial Direct Current Stimulation (tDCS), delivering a weak, constant electrical current to the dorsolateral prefrontal cortex (DLPFC) via a headset [29]
- Efficacy Data:
- Approval was based on a large, double-blind, sham-controlled randomized trial (N=174) recently published in Nature Medicine [26]
- Results:
- The active group showed a significantly greater reduction in Hamilton Depresion Rating Scale (HDRS) scores compared to sham (p≈.0.012;effect size 0.37)
- Response and remission were higher with active tDCS than sham, with approximate NNT=5 for response and =4 for remission
- Functional unblinding is a live concern in home neuromodulation trials, and FDA explicitly flags unblinding + mixed literature when weighing benefit.
- Practical Aspects:
- The device requires a prescription in the U.S. [25]
- Price Range: $500 – $800 (US estimate when it launches in the second half of 2026)
- In Europe it is already available for €459
- Protocol: Patients typically self-administer five 30-minute sessions per week for the first 3 weeks, followed by a maintenance schedule of three sessions per week [26]
- Side Effects: Generally mild, including skin redness/irritation at the electrode site and transient headaches. No serious adverse events were reported in trials [25]
Risperidone ER (Uzedy) – Bipolar I Disorder (October 10, 2025)
- The FDA expanded the label for Uzedy, a subcutaneous long-acting injectable (LAI) formulation of risperidone, to include the maintenance treatment of Bipolar I Disorder [30]
- Risperidone extended-release injectable suspension for subcutaneous use is indicated for use every one or two months for the treatment of schizophrenia in adults [31]
- New Indication:
- Monotherapy or adjunctive therapy to lithium or valproate for the maintenance treatment of Bipolar I Disorder in adults [30]
- Designed to address the high rates of non-adherence in bipolar disorder.
- The lack of oral lead-in may represent a logistical advantage in acute stabilization settings where oral adherence cannot be guaranteed
- Subcutaneous Injection:
- Administered under the skin (abdomen or upper arm), utilizing a smaller needle than traditional intramuscular gluteal/deltoid injections [30]
- Uses a copolymer technology that allows for a controlled, steady release of risperidone [31]
- No oral lead-in: Therapeutic plasma concentrations are reached within 6 to 24 hours of the first injection, eliminating the need for oral supplementation at initiation [31]
- Practical Aspects:
- Dosing Intervals: Available in monthly (every 1 month) or bi-monthly (every 2 months) dosing options (50 mg, 75 mg, 100 mg, 125 mg) [31]
- For bipolar maintenance, use the monthly regimen (the q2-month schedule is not recommended per labeling) [32]
Schizophrenia and Psychosis
Elimination of Clozapine REMS (February 24, 2025)
- The FDA eliminated the Clozapine Risk Evaluation and Mitigation Strategy (REMS) program, in a historic move designed to improve access to the “gold standard” for treatment-resistant schizophrenia [33]
- Although the risk of severe neutropenia with clozapine still exists, FDA has determined that the REMS program for clozapine is no longer necessary to ensure the benefits of the medicine outweigh that risk [34]
- Previously, the program required pharmacies to verify patient enrollment and Absolute Neutrophil Counts (ANC) in a centralized database before dispensing, often creating administrative barriers to care.
- Previously, the program required pharmacies to verify patient enrollment and Absolute Neutrophil Counts (ANC) in a centralized database before dispensing, often creating administrative barriers to care.
- New workflow:
- Prescribers:
- Must still monitor ANC according to the product labeling (e.g., weekly for first 6 months)
- However, they are no longer required to enroll patients in the REMS portal or report every ANC value to the FDA database to generate a “dispense authorization.”
- Pharmacies:
- Do not need to be enrolled in the REMS to order clozapine from wholesaler distributors.
- Pharmacists do not need to verify patient eligibility, including ANC monitoring, before dispensing clozapine to patients.
- Prescribers:
- Ongoing safety considerations:
- The boxed warning for severe neutropenia (agranulocytosis) remains in effect [35]
- Clinical responsibility for ANC monitoring now rests entirely with prescribers and their healthcare teams through internal protocols rather than federally mandated registry verification
- This removes the administrative “bottleneck” that often discouraged clinicians from prescribing clozapine.
Attention deficit hyperactivity disorder (ADHD)
Class-Wide Label Update: Extended-Release Stimulants (June 30, 2025)
- The FDA issued a Drug Safety Communication requiring updated warnings for all extended-release (ER) methylphenidate and amphetamine products [36]
- The updated labels must include a “Limitation of Use” stating that children <6 years have higher plasma exposures and higher rates of adverse reactions, including clinically significant weight loss [36]
- Findings were consistent across ER amphetamine and ER methylphenidate products, leading FDA to conclude that the benefit–risk profile does not support ER formulations in children <6.
- Rationale:
- While many ER stimulants are not FDA-approved for children under 6, off-label prescribing in this age group has increased [36]
- Clinical implications for prescribers:
- Avoid ER stimulants in preschoolers whenever possible. For children <6 who are currently on an ER formulation, reassess benefit–risk; if weight loss or other AEs emerge, FDA recommends stopping the ER product and/or switching to alternatives (e.g., IR stimulants, behavioral therapy) [36]
- For any child on stimulants, but especially those <6, monitor height, weight, and BMI percentiles regularly and intervene early if weight loss or growth deceleration occurs.
Alzheimer’s Disease
Donanemab (Kisunla) – Modified Titration Schedule (July 2025)
- The FDA approved an updated label for Kisunla (donanemab-azbt) that recommends a new, more gradual titration regimen [37]The FDA approved an updated label for Kisunla (donanemab-azbt) that recommends a new, more gradual titration regimen [37]
- Specifically intended to reduce the risk of amyloid-related imaging abnormalities with edema/effusion (ARIA-E) in patients with early symptomatic Alzheimer’s disease
- ARIA can be detected via magnetic resonance imaging (MRI) scans and may present as ARIA with edema (ARIA-E) and ARIA with hemosiderin deposition (ARIA-H) [38]
- ARIA is usually asymptomatic, although serious and life-threatening events can occur.
- ARIA is usually asymptomatic, although serious and life-threatening events can occur.
- Pharmacology:
- Donanemab is an IV anti–amyloid-β monoclonal antibody that binds deposited amyloid plaques and promotes their clearance, thereby slowing cognitive and functional decline in patients with mild cognitive impairment or mild dementia due to Alzheimer’s disease [39]
- Titration regimen
- Previous standard regimen: 700/700/700/1400 mg
- New titration regimen: The update replaces the standard rapid escalation with a more gradual step-up protocol to mitigate inflammatory responses during the initial treatment phase [38]
- Practical Aspects & Psychiatric Relevance:
- Kisunla is given as a 30-minute IV infusion every 4 weeks, following the new titration schedule and with serial brain MRI to monitor for ARIA.
- “Treat-to-clear” strategy from TRAILBLAZER-ALZ 2: clinicians can stop treatment once amyloid plaques are reduced to minimal levels on PET, rather than continuing indefinitely [40–43]
Lumipulse Plasma (Fujirebio) – First FDA-Cleared Blood Test (May 16, 2025)
- The FDA cleared for marketing the first in vitro diagnostic device that tests blood to aid in diagnosing Alzheimer’s disease [44]
- Indication:
- For use in adult patients aged 55 years and older presenting with cognitive impairment who are being evaluated for Alzheimer’s disease.
- Mechanism:
- The test measures the ratio of p-Tau217 (phosphorylated tau) to β-Amyloid 1-42 in blood plasma [LumipulseFDA]
- This ratio correlates with the presence or absence of amyloid plaques in the patient’s brain.
- Accuracy Data:
- In clinical validation studies involving 499 patients, the test demonstrated high concordance with the “gold standards” (PET scans or CSF analysis) [LumipulseFDA]
- Practical Aspects:
- Accessibility: Offers a less invasive, faster, and significantly cheaper alternative to PET imaging or lumbar puncture.
- However, the Lumipulse G pTau217/ß-Amyloid 1-42 Plasma Ratio is not intended as a screening or stand-alone diagnostic test, and other clinical evaluations or additional tests should be used for determining treatment options [44]
Addiction and Pain Management
Cyclobenzaprine Sublingual (Tonmya) – Fibromyalgia (August 15, 2025)
- Tonmya (cyclobenzaprine HCl sublingual tablets) received FDA approval for the management of fibromyalgia [45]
- Indication: Management of fibromyalgia in adults
- Pharmacology & Mechanism:
- Tonmya is a sublingual, transmucosal formulation of cyclobenzaprine (2.8 mg or 5.6 mg) designed for rapid absorption at bedtime.
- The sublingual route bypasses first-pass hepatic metabolism, leading to higher parent cyclobenzaprine and lower norcyclobenzaprine exposure than oral tablets [46]
- Norcyclobenzaprine is the active metabolite of cyclobenzapirine, with a half-life of several days (approximately 72hours)
- This is intended to reduce next-day grogginess and prolonged sedation
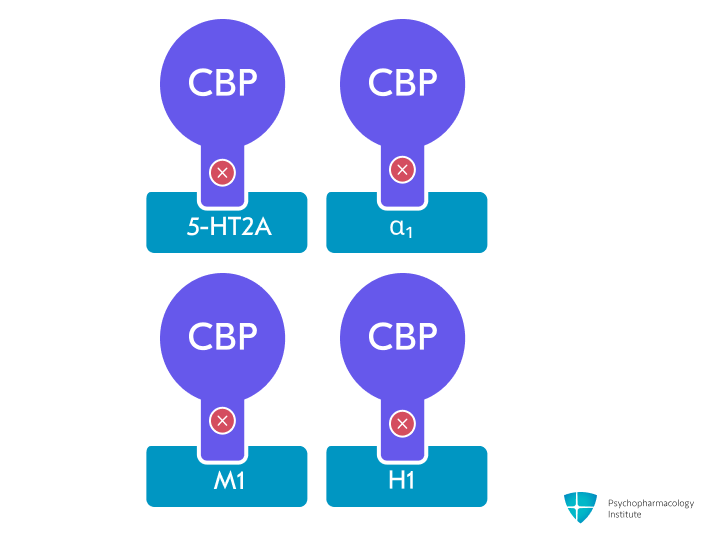
- Cyclobenzaprine antagonizes 5-HT₂A, α₁-adrenergic, M₁-muscarinic, and H₁-histaminergic receptors, modulating sleep architecture and decreasing nonrestorative sleep, which is strongly linked to central sensitization and pain amplification in fibromyalgia [46]
- Norcyclobenzaprine antagonizes the same receptors as cyclobenzaprine but with lower potency
- Norcyclobenzaprine is also a more potent inhibitor of norepinephrine transporters than cyclobenzaprine
- Conceptually, Tonmya is positioned as a centrally acting, sleep-targeted analgesic that may interrupt the cycle of poor sleep → heightened pain → further sleep disruption [46]
- Efficacy data:
- Effect size for pain reduction was ~0.38, similar to duloxetine, pregabalin, and milnacipran (with effect sizes of 0.36, 0.31, and 0.22, respectively) [46–49]
- Safety & Tolerability:
- Overall tolerability was favorable; most adverse events were mild, transient oral effects related to sublingual administration (oral hypoesthesia, paresthesia, altered taste) [46,50]
- Rates of serious adverse events and discontinuations due to AEs were low and comparable to placebo in the Phase 3 program.
- Practical Aspects & Relevance:
- Dosing: Taken once nightly at bedtime, typically after a short titration period (2.8 mg for 2 weeks, then 5.6 mg)
- While cyclobenzaprine is not a novel molecule, FDA approval of this low-dose sublingual formulation will enable broader clinical use. Real-world evidence from post-marketing experience will clarify where it fits within the fibromyalgia treatment landscape [51]
Sublocade (Buprenorphine Extended-Release) – Rapid Initiation (February 24, 2025)
- FDA approved a label update for buprenorphine extended-release injection (Sublocade) that introduces a rapid initiation protocol and alternative injection sites for the treatment of moderate-to-severe opioid use disorder (OUD) [52]
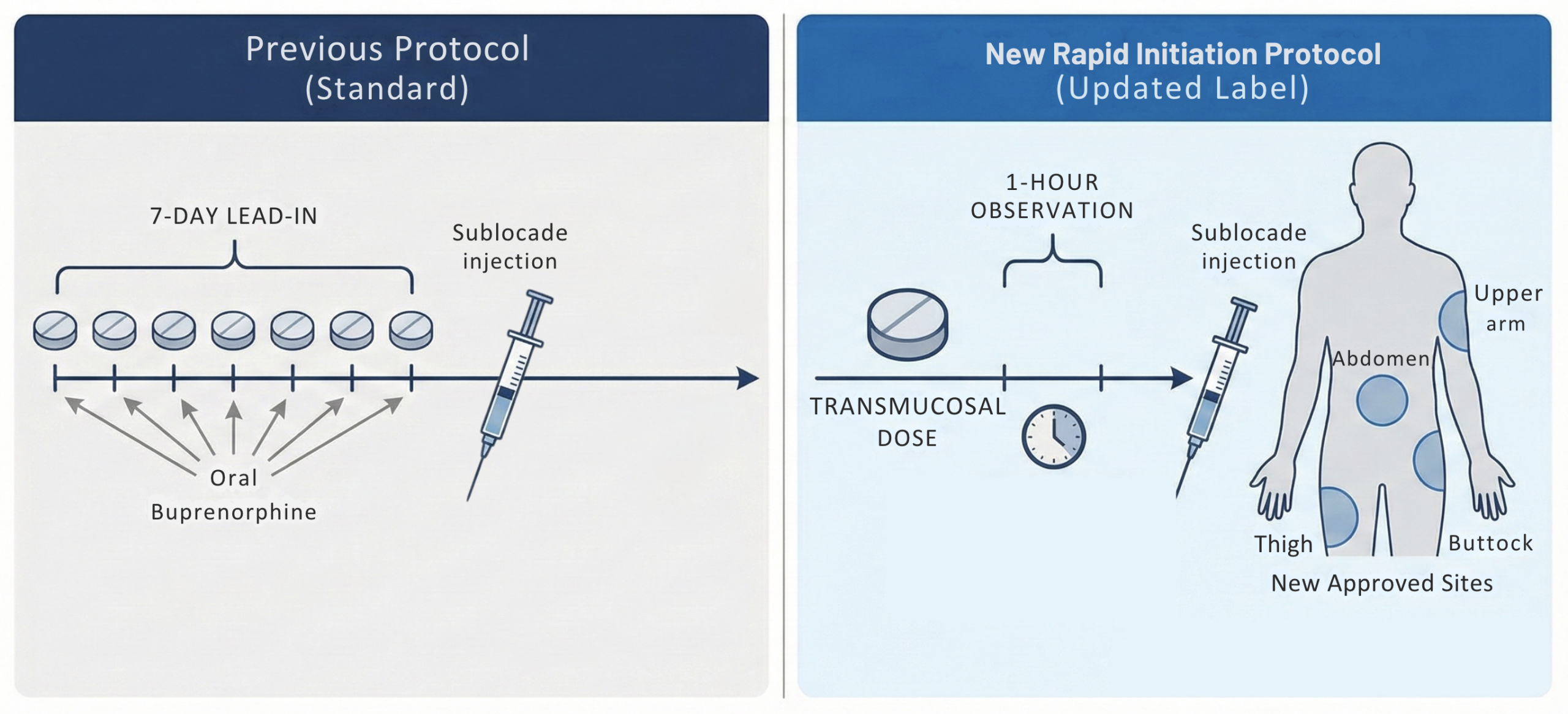
- The previous label required a 7-day “lead-in” period with oral transmucosal buprenorphine to confirm tolerability/clinical appropriateness. This week-long gap often resulted in patient dropout, particularly in emergency department (ED) or crisis settings [53]
- New label: Allows for the administration of the Sublocade injection after just a single dose of transmucosal buprenorphine, followed by a 1-hour observation period prior to injection [52]
- New Injection Sites: The approval also expands administration sites to include the thigh, buttock, and upper arm (previously restricted to the abdomen) [52]
- This added flexibility can be helpful in patients with central obesity, scarring, or prior injection-site issues, and may facilitate administration across diverse clinical settings.
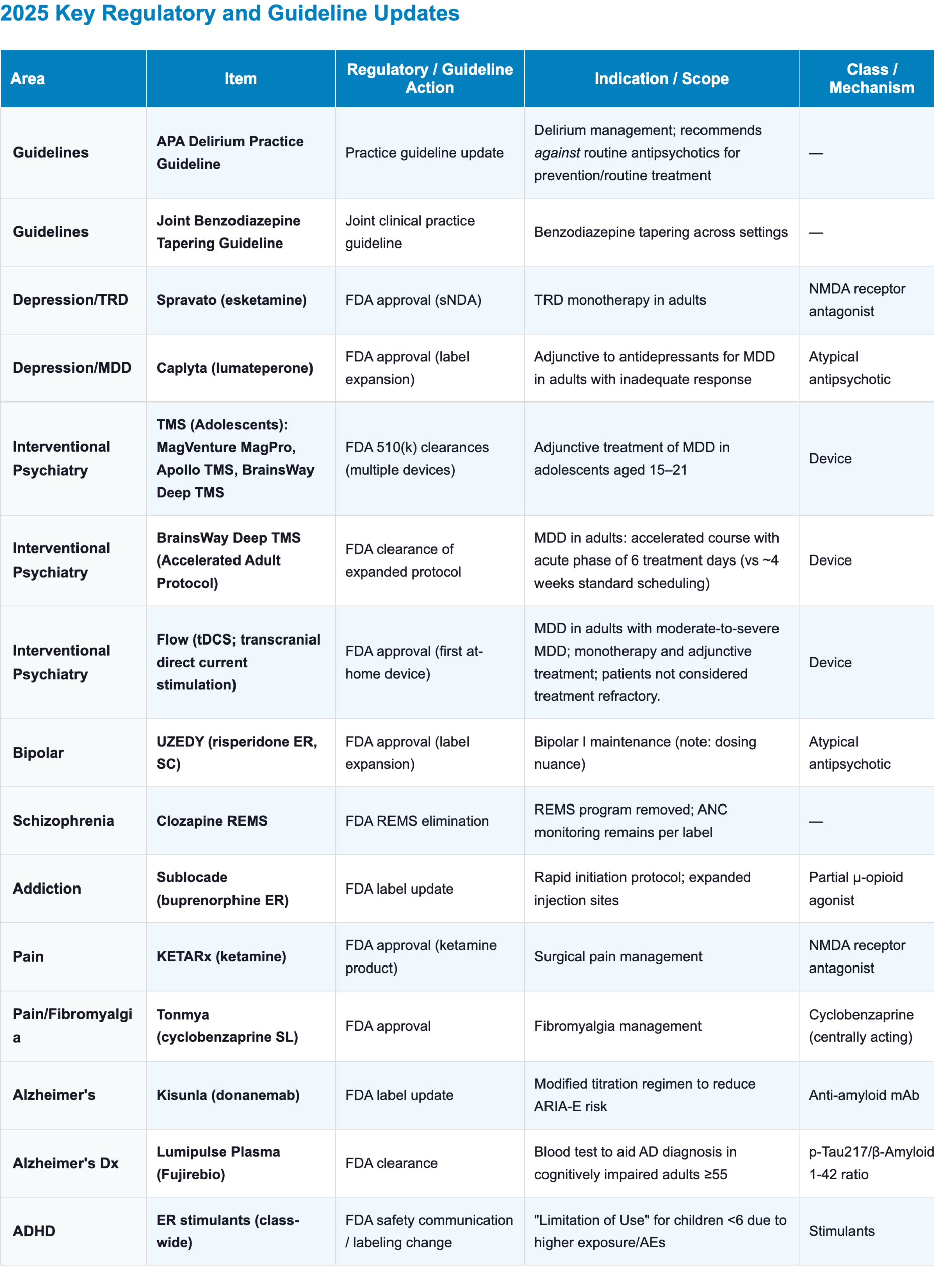
Appendix: 2025 Key Regulatory and Guideline Updates
References
1. The american psychiatric association practice guideline for the prevention and treatment of delirium (Second Edition). (2025). American Psychiatric Publishing. https://doi.org/10.1176/appi.books.9780890428023
2. Crone, C., Fochtmann, L. J., Ahmed, I., Balas, M. C., Boland, R., Escobar, J. I., Heinrich, T., Jackson-Triche, M., Levenson, J. L., Mattison, M., McCullen Truett, J., Oldham, M. A., Seritan, A., Fochtmann, L. J., & Hong, S.-H. (2025). The American Psychiatric Association Practice Guideline for the Prevention and Treatment of Delirium. American Journal of Psychiatry, 182(9), 880–884. https://doi.org/10.1176/appi.ajp.25182013
3. CGC, & ASAM. (2025). Joint CPG on BZD Tapering: Considerations when Benzodiazepine Risks Outweigh Benefits. https://downloads.asam.org/sitefinity-production-blobs/docs/default-source/guidelines/benzodiazepine-tapering-2025/bzd-tapering-document—final-approved-version-for-distribution-02-28-25.pdf?sfvrsn=5bdf9c81_6
4. US Food and Drug Administration (2025). SPRAVATOreg esketamine hydrochloride solution Janssen Pharmaceuticals Inc. Prescribing Information.
5. Janik, A., Qiu, X., Lane, R., Popova, V., Drevets, W. C., Canuso, C. M., Macaluso, M., Mattingly, G. W., Shelton, R. C., Zajecka, J. M., & Fu, D.-J. (2025). Esketamine Monotherapy in Adults With Treatment-Resistant Depression: A Randomized Clinical Trial. JAMA Psychiatry, 82(9), 877–887. https://doi.org/10.1001/jamapsychiatry.2025.1317
6. Sanacora, G., Ahmed, M., Brown, B., Cabrera, P., Doherty, T., Himedan, M., Kern, D. M., Lim, L., Lopena, O., Ronaldo R. Naranjo, J., Nuamah, I., Sarayani, A., Turkoz, I., & Bowrey, H. E. (2025). Real-World Safety of Esketamine Nasal Spray: A Comprehensive Analysis Almost 5 Years After First Approval. American Journal of Psychiatry. https://doi.org/10.1176/appi.ajp.20240655
7. Naudet, F., Pellen, C., Fodor, L. A., Gastaldon, C., Barbui, C., Turner, E. H., Le Pabic, E., & Cristea, I. A. (2025). Efficacy and safety of esketamine for “treatment resistant depression”: Registered report for a systematic review with an individual patient data meta-analysis of randomized, double-blind, placebo-controlled trials. BMC Medicine, 23(1), 677. https://doi.org/10.1186/s12916-025-04435-x
8. Vasiliu, O. (2023). Esketamine for treatment-resistant depression: A review of clinical evidence (Review). Experimental and Therapeutic Medicine, 25(3), 111. https://doi.org/10.3892/etm.2023.11810
9. Papakostas, G. I., Salloum, N. C., Hock, R. S., Jha, M. K., Murrough, J. W., Mathew, S. J., Iosifescu, D. V., & Fava, M. (2020). Efficacy of Esketamine Augmentation in Major Depressive Disorder: A Meta-Analysis. The Journal of Clinical Psychiatry, 81(4), 19r12889. https://doi.org/10.4088/JCP.19r12889
10. Floriano, I., Silvinato, A., & Bernardo, W. M. (2023). The use of esketamine in the treatment of patients with severe depression and suicidal ideation: Systematic review and meta-analysis. Revista Da Associação Médica Brasileira, 69(4), e2023D694. https://doi.org/10.1590/1806-9282.2023d694
11. McIntyre, R. S., Rosenblat, J. D., Nemeroff, C. B., Sanacora, G., Murrough, J. W., Berk, M., Brietzke, E., Dodd, S., Gorwood, P., Ho, R., Iosifescu, D. V., Lopez Jaramillo, C., Kasper, S., Kratiuk, K., Lee, J. G., Lee, Y., Lui, L. M. W., Mansur, R. B., Papakostas, G. I., … Stahl, S. (2021). Synthesizing the Evidence for Ketamine and Esketamine in Treatment-Resistant Depression: An International Expert Opinion on the Available Evidence and Implementation. The American Journal of Psychiatry, 178(5), 383–399. https://doi.org/10.1176/appi.ajp.2020.20081251
12. Cristea, I. A., Plöderl, M., & Naudet, F. (2025). Esketamine for Treatment-Resistant Depression. JAMA Psychiatry. https://doi.org/10.1001/jamapsychiatry.2025.3242
13. FDA approval of CAPLYTAreg (lumateperone) has the potential to reset treatment expectations, offering hope for remission in adults with major depressive disorder. (2025, November 6). JNJ.com. https://www.jnj.com/media-center/press-releases/fda-approval-of-caplyta-lumateperone-has-the-potential-to-reset-treatment-expectations-offering-hope-for-remission-in-adults-with-major-depressive-disorder
14. US Food and Drug Administration (2025). CAPLYTAreg (lumateperone) capsules, for oral use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/209500s016lbl.pdf
15. IsHak, W. W., Hirsch, D., Renteria, S., Totlani, J., Murphy, N., Chang, T., Abdelsalam, R., Salem, M., Meyer, A., Keerthana, S., Liu, A., Contreras, L., Tadros, E., Hedrick, R., Danovitch, I., & Pechnick, R. N. (2025). Depressive disorders: Systematic review of approved psychiatric medications (2009-April 2025) and pipeline phase 3 medications. BMC Psychiatry, 25, 939. https://doi.org/10.1186/s12888-025-07141-3
16. Jha, M. K., & Mathew, S. J. (2023). Pharmacotherapies for Treatment-Resistant Depression: How Antipsychotics Fit in the Rapidly Evolving Therapeutic Landscape. American Journal of Psychiatry. https://doi.org/10.1176/appi.ajp.20230025
17. Thase, M. (n.d.). Adjunctive Antipsychotic Therapies in the Treatment of Major Depressive Disorder. Psychiatry Advisor. Retrieved December 8, 2025, from https://www.psychiatryadvisor.com/cch/major-depressive-disorder-second-generation-antipsychotics-brexpiprazole/
18. Durgam, S., Earley, W., Kozauer, S. G., Chen, C., Lakkis, H., Edwards, J. B., & Stahl, S. (2024). Lumateperone as adjunctive therapy in patients with major depressive disorder: Results from a randomized, double-blind, phase 3 trial. Neuroscience Applied, 3, 104586. https://doi.org/10.1016/j.nsa.2024.104586
19. Durgam, S., Earley, W. R., Kozauer, S. G., Chen, C., Edwards, J. B., Jain, R., & Correll, C. U. (n.d.). Adjunctive Lumateperone 42 mg in the Treatment of Major Depressive Disorder: A Pooled Analysis of 2 Phase 3 Randomized Controlled Trials.
20. Neuronetics. (2024, March 25). NeuroStarreg Advanced Therapy Receives FDA Clearance as a First-Line Add-On Treatment for Adolescents with Depression. GlobeNewswire News Room. https://www.globenewswire.com/en/news-release/2024/03/25/2851611/0/en/NeuroStar-Advanced-Therapy-Receives-FDA-Clearance-as-a-First-Line-Add-On-Treatment-for-Adolescents-with-Depression.html
21. Mandeville, R. (2025, August 25). FDA clearance of TMS Therapy for Adolescents. MagVenture US. https://magventure.com/us/fda-clearance-to-expand-tms-therapy-indication-for-adolescents-aged-15-21/
22. AG, neurocare group. (2025, September 10). Neurocare group’s Apollo TMS Therapy Devices Now Have FDA Clearance to Treat Adolescents Suffering With Major Depression. GlobeNewswire News Room. https://www.globenewswire.com/news-release/2025/09/10/3147821/0/en/neurocare-group-s-Apollo-TMS-Therapy-Devices-Now-Have-FDA-Clearance-to-Treat-Adolescents-Suffering-With-Major-Depression.html
23. BrainsWay Receives FDA Clearance of Deep TMSTM as Adjunct Therapy for Major Depressive Disorder (MDD) in Adolescents Aged 15 to 21 – BrainsWay. (n.d.). Retrieved December 8, 2025, from https://investors.brainsway.com/news-releases/news-release-details/brainsway-receives-fda-clearance-deep-tmstm-adjunct-therapy/
24. Hanlon, C. A., Roth, Y., Bermudes, R. A., Brink, E., Davis, M., DeBrocco, D., Ellis, S., Jones, L., Khan, A., MacMillan, C., Mudunuru, A. K., Muir, O., Pell, G. S., Prestley, T., Reddy, M. S., Sisko, E., Seplow, S., Slomowitz, N., Stein, A., … Tendler, A. (2025, October 22). Accelerated Deep TMS for Depression: Results from a Multisite, Randomized Non-Inferiority Trial (SSRN Scholarly Paper 5583382). https://doi.org/10.2139/ssrn.5583382 (Pre-published)
25. FDA Approves World’s First At-Home Brain Stimulation Treatment for Depression. (2025, December 11). https://www.flowneuroscience.com/fda-approved-lp-2/
26. Woodham, R. D., Selvaraj, S., Lajmi, N., Hobday, H., Sheehan, G., Ghazi-Noori, A.-R., Lagerberg, P. J., Rizvi, M., Kwon, S. S., Orhii, P., Maislin, D., Hernandez, L., Machado-Vieira, R., Soares, J. C., Young, A. H., & Fu, C. H. Y. (2025). Home-based transcranial direct current stimulation treatment for major depressive disorder: A fully remote phase 2 randomized sham-controlled trial. Nature Medicine, 31(1), 87–95. https://doi.org/10.1038/s41591-024-03305-y
27. Woodham, R., Rimmer, R. M., Mutz, J., & Fu, C. H. Y. (2021). Is tDCS a potential first line treatment for major depression? International Review of Psychiatry (Abingdon, England), 33(3), 250–265. https://doi.org/10.1080/09540261.2021.1879030
28. Ironside, M., Browning, M., Ansari, T. L., Harvey, C. J., Sekyi-Djan, M. N., Bishop, S. J., Harmer, C. J., & O’Shea, J. (2019). Effect of Prefrontal Cortex Stimulation on Regulation of Amygdala Response to Threat in Individuals With Trait Anxiety: A Randomized Clinical Trial. JAMA Psychiatry, 76(1), 71–78. https://doi.org/10.1001/jamapsychiatry.2018.2172
29. Keeser, D., Meindl, T., Bor, J., Palm, U., Pogarell, O., Mulert, C., Brunelin, J., Möller, H.-J., Reiser, M., & Padberg, F. (2011). Prefrontal transcranial direct current stimulation changes connectivity of resting-state networks during fMRI. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 31(43), 15284–15293. https://doi.org/10.1523/JNEUROSCI.0542-11.2011
30. FDA Approves Expanded Indication for UZEDYreg (risperidone) Extended-Release Injectable Suspension as a Treatment for Adults Living with Bipolar I Disorder. (n.d.). Retrieved December 8, 2025, from https://ir.tevapharm.com/news-and-events/press-releases/press-release-details/2025/FDA-Approves-Expanded-Indication-for-UZEDY-risperidone-Extended-Release-Injectable-Suspension-as-a-Treatment-for-Adults-Living-with-Bipolar-I-Disorder/default.aspx
31. US Food and Drug Administration (2025). UZEDY (risperidone) extended-release injectable suspension, for subcutaneous use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/213586s008s009lbl.pdf
32. US Food and Drug Administration (2025). UZEDYreg (risperidone) extended-release injectable suspension. https://www.uzedy.com/globalassets/uzedy/prescribing-information.pdf#xd_co_f=MGJhYmRmNDEtNmU1Ni00ZjdhLTk4MWQtOWZiNzcxNjA0NzA0~
33. US Food and Drug Administration (Wed, 08/27/2025 – 14:52). FDA removes risk evaluation and mitigation strategy (REMS) program for the antipsychotic drug Clozapine. FDA. https://www.fda.gov/drugs/drug-safety-and-availability/fda-removes-risk-evaluation-and-mitigation-strategy-rems-program-antipsychotic-drug-clozapine
34. US Food and Drug Administration (Tue, 02/25/2025 – 09:29, Tue, 02/25/2025 – 09:29). Frequently Asked Questions | Clozapine REMS Modification. Clozapine REMS Modification; FDA. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/frequently-asked-questions-clozapine-rems-modification
35. CLOZARILreg (clozapine) tablets, for oral use. (n.d.). Retrieved December 8, 2025, from https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/019758s106lbl.pdf
36. US Food and Drug Administration (Tue, 07/01/2025 – 11:32). FDA requires expanded labeling about weight loss risk in patients younger than 6 years taking extended-release stimulants for ADHD. FDA. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-expanded-labeling-about-weight-loss-risk-patients-younger-6-years-taking-extended
37. Company, E. L. and. (2025, July 9). FDA approves updated label for Lilly’s Kisunla (donanemab-azbt) with new dosing in early symptomatic Alzheimer’s disease. https://investor.lilly.com/news-releases/news-release-details/fda-approves-updated-label-lillys-kisunla-donanemab-azbt-new
38. US Food and Drug Administration (2025). KISUNLA (donanemab-azbt) injection, for intravenous use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2025/761248s004lbl.pdf
39. Rabinovici, G. D., Selkoe, D. J., Schindler, S. E., Aisen, P., Apostolova, L. G., Atri, A., Greenberg, S. M., Hendrix, S. B., Petersen, R. C., Weiner, M., Salloway, S., & Cummings, J. (2025). Donanemab: Appropriate use recommendations. The Journal of Prevention of Alzheimer’s Disease, 12(5), 100150. https://doi.org/10.1016/j.tjpad.2025.100150
40. Sims, J. R., Zimmer, J. A., Evans, C. D., Lu, M., Ardayfio, P., Sparks, J., Wessels, A. M., Shcherbinin, S., Wang, H., Monkul Nery, E. S., Collins, E. C., Solomon, P., Salloway, S., Apostolova, L. G., Hansson, O., Ritchie, C., Brooks, D. A., Mintun, M., Skovronsky, D. M., & TRAILBLAZER-ALZ 2 Investigators. (2023). Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA, 330(6), 512–527. https://doi.org/10.1001/jama.2023.13239
41. Ross, E. L., Weinberg, M. S., & Arnold, S. E. (2022). Cost-effectiveness of Aducanumab and Donanemab for Early Alzheimer Disease in the US. JAMA Neurology, 79(5), 478–487. https://doi.org/10.1001/jamaneurol.2022.0315
42. Boustani, M., Doty, E. G., Garrison, L. P., Smolen, L. J., Belger, M., Klein, T. M., Murphy, D. R., Burge, R., Wall, J. K., & Johnston, J. A. (2022). Assessing the Cost-effectiveness of a Hypothetical Disease-modifying Therapy With Limited Duration for the Treatment of Early Symptomatic Alzheimer Disease. Clinical Therapeutics, 44(11), 1449–1462. https://doi.org/10.1016/j.clinthera.2022.09.008
43. Mattke, S., Ozawa, T., & Hanson, M. (2024). Implications of treatment duration and frequency for value and cost-effective price of Alzheimer treatments. Journal of Managed Care & Specialty Pharmacy, 30(10), 1087–1094. https://doi.org/10.18553/jmcp.2024.24116
44. Office of the Commissioner. US Food and Drug Administration (Wed, 05/28/2025 – 11:33, Wed, 05/28/2025 – 11:33). FDA Clears First Blood Test Used in Diagnosing Alzheimer’s Disease. FDA Clears First Blood Test Used in Diagnosing Alzheimer’s Disease; FDA. https://www.fda.gov/news-events/press-announcements/fda-clears-first-blood-test-used-diagnosing-alzheimers-disease
45. Tonix Pharmaceuticals (2025). Tonix Pharmaceuticals Announces FDA Approval of TonmyaTM (cyclobenzaprine HCl sublingual tablets) for the Treatment of Fibromyalgia. https://ir.tonixpharma.com/news-events/press-releases/detail/1585/tonix-pharmaceuticals-announces-fda-approval-of
46. Lederman, S., Arnold, L. M., Vaughn, B., Engels, J. M., Kelley, M., & Sullivan, G. M. (2025). Pain relief by targeting nonrestorative sleep in fibromyalgia: A phase 3 randomized trial of bedtime sublingual cyclobenzaprine. Pain Medicine, pnaf089. https://doi.org/10.1093/pm/pnaf089
47. Perrot, S., & Russell, I. j. (2014). More ubiquitous effects from non-pharmacologic than from pharmacologic treatments for fibromyalgia syndrome: A meta-analysis examining six core symptoms. European Journal of Pain, 18(8), 1067–1080. https://doi.org/10.1002/ejp.564
48. Häuser, W., Petzke, F., & Sommer, C. (2010). Comparative Efficacy and Harms of Duloxetine, Milnacipran, and Pregabalin in Fibromyalgia Syndrome. The Journal of Pain, 11(6), 505–521. https://doi.org/10.1016/j.jpain.2010.01.002
49. Farag, H. M., Yunusa, I., Goswami, H., Sultan, I., Doucette, J. A., & Eguale, T. (2022). Comparison of Amitriptyline and US Food and Drug Administration–Approved Treatments for Fibromyalgia. JAMA Network Open, 5(5), e2212939. https://doi.org/10.1001/jamanetworkopen.2022.12939
50. Lederman, S., Arnold, L. M., Vaughn, B., Kelley, M., & Sullivan, G. M. (2023). Efficacy and Safety of Sublingual Cyclobenzaprine for the Treatment of Fibromyalgia: Results From a Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Care & Research, 75(11), 2359–2368. https://doi.org/10.1002/acr.25142
51. Kaufman, M. B., PharmD, & BCGP. (n.d.). A New Treatment for Fibromyalgia? – Page 3 of 4. The Rheumatologist. Retrieved December 9, 2025, from https://www.the-rheumatologist.org/article/a-new-treatment-for-fibromyalgia/
52. Indivior. (2025, February 24). Indivior Announces FDA Approval of Label Changes for SUBLOCADEreg (buprenorphine extended-release) Injection. https://www.indivior.com/en/media/press-releases/indivior-announces-fda-approval-of-label-changes-for-sublocade-injection
53. US Food and Drug Administration (2023). SUBLOCADE (buprenorphine extended-release) injection, for subcutaneous use, CIII. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/209819s028lbl.pdf



