In a nutshell

- Dose-dependent effects:
- Venlafaxine’s efficacy increases with higher doses, but this comes at the cost of decreased acceptability and tolerability1.
- Higher doses are associated with elevated blood pressure.
- Efficacy for anhedonia and fatigue:
- Venlafaxine may help patients with anhedonia and fatigue, likely due to its effects on norepinephrine and dopamine pathways2.
- High risk of discontinuation syndrome:
- Venlafaxine has a higher risk of discontinuation syndrome than other antidepressants3.
Pharmacodynamics and mechanism of action
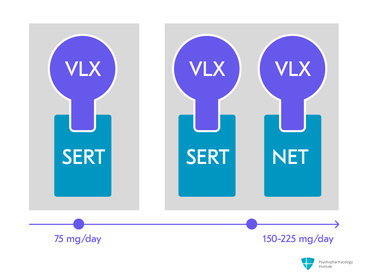
- Venlafaxine’s mechanism of action appears to be dose-dependent:
- At lower doses (37.5-75 mg/day): serotonin reuptake inhibition.
- At moderate doses (150−225 mg/day): dual mechanism agent affecting serotonin and norepinephrine4,5.
Pharmacokinetics
Metabolism
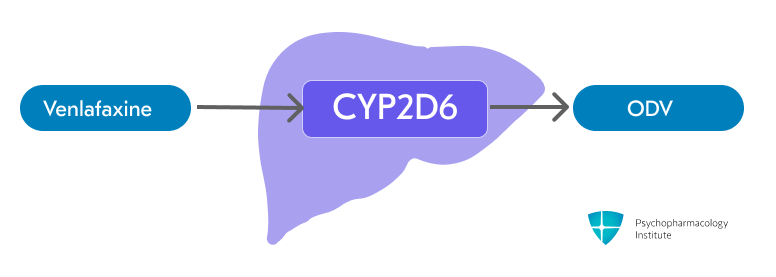
Venlaxafine is metabolized to O-desmethylvenlafaxine (ODV)
- Venlafaxine is primarily metabolized to its active metabolite, O-desmethylvenlafaxine (ODV), via CYP2D6.
- CYP3A4 is involved in the metabolism of venlafaxine, though to a lesser extent than CYP2D6.6
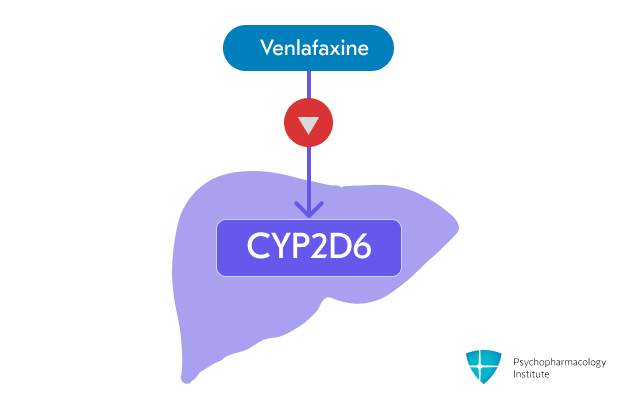
Venlafaxine is a weak inhibitor of CYP2D6
- Venlafaxine is a weak inhibitor of CYP2D6
- It may increase the concentrations of CYP2D6 substrates
- Caution when used with other drugs metabolized by CYP2D6
- Drug interactions:
- Contraindicated with MAOIs
- CYP3A4 inhibitors may increase venlafaxine and ODV levels
Half-life
- Venlafaxine and its active metabolite O-desmethylvenlafaxine (ODV) have different half-lives.
- Venlafaxine: 5 hours
- O-desmethylvenlafaxine (ODV): 11 hours
Dosage forms
- Immediate-release:
- Tablets:
- 25 mg, 37.5 mg, 50 mg, 75 mg, 100 mg
- Generic
- Tablets:
- Extended-release:
- Capsules:
- 37.5 mg, 75 mg, 150 mg
- Generic, Effexor XR
- Tablets:
- 37.5 mg, 75 mg, 150 mg, 225 mg
- Generic
- Tablets (besylate salt):
- 112.5 mg
- Generic
- Capsules:
Indications
FDA-Approved Indications
Major depressive disorder
- Guidelines recommend venlafaxine-XR as a first-line pharmacotherapy for major depression7.
- Dose-dependent action:
- At lower doses (37.5 –75 mg/day), venlafaxine can be considered to act as an SSRI primarily.
- At higher doses (150-225 mg/day), its noradrenergic effects can help treat the anhedonia/fatigue symptom cluster.
- Venlafaxine is an option to switch to if patients do not adequately respond to the initial antidepressant prescribed8.
- Dosing
- Starting dose: 37.5 –75 mg/day.
- For most patients, 75 mg/day.
- Target dose: 75 mg/day
- Increments: no faster than 75 mg every 4 days.
- Maximum recommended dose: 225 mg/day
- Formulation considerations:
- XR capsules and tablets: taken once daily, with food.
- IR tablets can be divided into 2-3 daily doses.
- If XR formulations are broken, the extended-release properties will be altered.
Generalized anxiety disorder
- SSRIs and SNRIs are first-line pharmacotherapies for GAD9.
- Dosing:
- Starting dose:
- For most patients, 75 mg/day
- For some patients, 37.5 mg/day
- Target dose: 75 mg/day
- Increments: no faster than 75 mg every 4 days.
- Maximum recommended dose: 225 mg/day
- Starting dose:
Social anxiety disorder
- Venlafaxine is a first-line drug for the treatment of SAD .
- Venlafaxine and paroxetine are similarly effective, according to a comparison trial10.
- Similar discontinuation rates, but paroxetine had better tolerability, with fewer adverse events requiring dose adjustment.
- Dosing:
- Starting dose: 75 mg/day
- Target dose: 75 mg/day
- Maximum dose: 75 mg/day (no benefit at higher doses)
Panic disorder
- Venlafaxine and SSRIs are first-line pharmacotherapies for panic disorder .
- Dosing
- Starting dose: 37.5 mg/day for 7 days
- Target dose: 75 mg/day
- Maximum dose: 225 mg/day
Off-label uses
PTSD
- When pharmacotherapy is needed, guidelines recommend starting with SSRIs such as sertraline or citalopram11.
- SNRIs such as venlafaxine are considered a reasonable first-line option12.
PMDD
- Venlafaxine has shown efficacy for the treatment of PMDD:
- as a continuous dosing strategy (open-label trial)13
- as luteal phase dosing strategy14
Side Effects
Most common side effects
Gastrointestinal
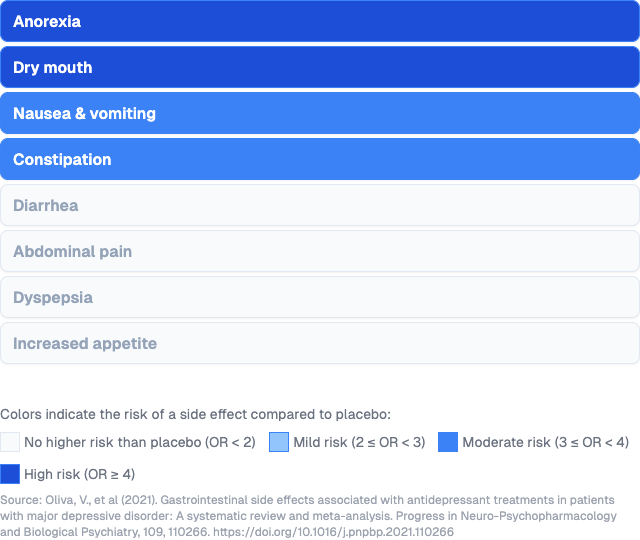
Gastrointestinal side effects
- Anorexia
- Dry mouth
- Nausea
- Reassure your patient that it is not dangerous and usually improves over time.
- Recommend ginger root in some form to alleviate nausea15.
- Start low, with half the intended dose
- Constipation
Other side effects
- Discontinuation syndrome
- Increased incidence at higher dosages, possibly related to noradrenergic effects.
- SNRIs, paroxetine, and mirtazapine have the highest risk among antidepressants.
- Antidepressant-induced sexual dysfunction
- Ranking of risk: SSRIs and venlafaxine > tricyclics > other SNRIs16.
- Headache
- Dizziness
- Nervousness
- Sleep alterations
- Decrease in REM sleep17.
- Somnolence
- Insomnia
- Sweating
- Dose-related side effect
- If antidepressant dose reduction is clinically feasible, it should be tried18.
Serious side effects
- Dose-related hypertension
- The extended-release formulation has a lower risk of diastolic hypertension19.
- Immediate-release: 10-15% of patients.
- Extended-release: almost 6% of patients.
- Chronic venlafaxine treatment can facilitate the occurrence of hypertensive crises in normotensive patients.
- The extended-release formulation has a lower risk of diastolic hypertension19.
- Hyponatremia
- Ranking of risk:
- MAOIs > SNRIs > SSRIs > TCAs > Mirtazapine20
- For hyponatremia-prone patients:
- Mirtazapine should be considered the antidepressant of choice.
- SNRIs should be prescribed more cautiously than SSRIs
- Ranking of risk:
Use in special populations
Breastfeeding
- Infants’ exposure through breastmilk21
- Infants receive venlafaxine.
- Side effects in breastfed infants have rarely been reported.
- Breastfed infants should be monitored for:
- Excessive sedation
- Adequate weight gain
- A safety scoring system finds venlafaxine use to be possible during breastfeeding22.
- However, some experts do not recommend venlafaxine during nursing23.
Hepatic impairment
- Mild (Child-Pugh Class A, score 5-6)
- Reduce total daily dose by 50%
- Moderate (Child-Pugh Class B, score 7-8)
- Reduce total daily dose by 50%
- Severe (Child-Pugh Class C, score 10-15) or cirrhosis
- Reduce the total daily dose by 50% or more
Renal impairment
- Mild (CLcr 60–89 mL/min)
- Reduce total daily dose by 25-50%
- Moderate (CLcr 30–59 mL/min)
- Reduce total daily dose by 25-50%
- Severe (CLcr <30 mL/min) or undergoing hemodialysis
- Reduce the total daily dose by 50% or more
Elderly
No dose adjustment is needed.
Brand names
- US: Effexor XR
- Canada: Effexor XR
- Other countries/regions: Alventa, Arafaxina, Argofan, Conervin, Deprevix, Dislaven, Dobupal, Duofaxin, Eduxon, Efectin, Efexor, Efevel, Elafax, Elify, Faxine, Faxipaw, Faxiprol, Flavix, Ganavax, Idixor, Jarvis, Lafaxin, Lanvexin, Laroxin, Maxibral, Melocin, Niztec, Norafexine, Norezor, Norpilen, Politid, Pracet, Pramina, Psiseven, Quilarex, Senexon, Serosmine, Sesaren, Sunvex, Tifaxin, Trevilor, Vandral, Vaxor, Velafax, Velaxin, Velostad, Velpine, Vendep, Venexor, Veniba, Veniz, Venlalic, Venxin, Venxor, Viepax, Vilfax, Voxafen, Voxatin, Zacalen, Zarelis, Zaxine
References
- Rush, A., Trivedi, M., Wisniewski, S., Nierenberg, A., Stewart, J., Warden, D., Niederehe, G., Thase, M., Lavori, P., Lebowitz, B., McGrath, P., Rosenbaum, J., Sackeim, H., Kupfer, D., Luther, J., & Fava, M. (2006). Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. New England Journal of Medicine, 354, 1231–1242
- Murawiec, S., & Krzystanek, M. (2021). Symptom Cluster-Matching Antidepressant Treatment: A Case Series Pilot Study. Pharmaceuticals, 14(6), 526. https://doi.org/10.3390/ph14060526
- Horowitz, M. A., Framer, A., Hengartner, M. P., Sørensen, A., & Taylor, D. (2023). Estimating Risk of Antidepressant Withdrawal from a Review of Published Data. CNS Drugs, 37(2), 143–157. https://doi.org/10.1007/s40263-022-00960-y
- Harvey, A. T., Rudolph, R. L., & Preskorn, S. H. (2000). Evidence of the Dual Mechanisms of Action of Venlafaxine. Archives of General Psychiatry, 57(5), 503. https://doi.org/10.1001/archpsyc.57.5.503
- Fagiolini, A., Cardoner, N., Pirildar, S., Ittsakul, P., Ng, B., Duailibi, K., & El Hindy, N. (2023). Moving from serotonin to serotonin-norepinephrine enhancement with increasing venlafaxine dose: Clinical implications and strategies for a successful outcome in major depressive disorder. Expert Opinion on Pharmacotherapy, 24(15), 1715–1723. https://doi.org/10.1080/14656566.2023.2242264
- Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc. (2022). EFFEXOR XR- venlafaxine hydrochloride capsule, extended release: Prescribing information. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=53c3e7ac-1852-4d70-d2b6-4fca819acf26
- Lam, R. W., Kennedy, S. H., Adams, C., Bahji, A., Beaulieu, S., Bhat, V., Blier, P., Blumberger, D. M., Brietzke, E., Chakrabarty, T., et al. (2024). Canadian network for mood and anxiety treatments (CANMAT) 2023 update on clinical guidelines for management of major depressive disorder in adults. The Canadian Journal of Psychiatry, 69(9), 641–687. https://doi.org/10.1177/07067437241245384
- The Management of Major Depressive Disorder Work Group. (2022). Management of Major Depressive Disorder (MDD) (2022). Department of Veterans Affairs and Department of Defense. https://www.healthquality.va.gov/guidelines/MH/mdd/
- Bandelow, B., Allgulander, C., Baldwin, D. S., Costa, D. L. D. C., Denys, D., Dilbaz, N., Domschke, K., Eriksson, E., Fineberg, N. A., Hättenschwiler, J., Hollander, E., Kaiya, H., Karavaeva, T., Kasper, S., Katzman, M., Kim, Y.-K., Inoue, T., Lim, L., Masdrakis, V., … Zohar, J. (2023). World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders – Version 3. Part I: Anxiety disorders. The World Journal of Biological Psychiatry, 24(2), 79–117. https://doi.org/10.1080/15622975.2022.2086295
- Liebowitz, M. R., Gelenberg, A. J., & Munjack, D. (2005). Venlafaxine extended release vs placebo and paroxetine in social anxiety disorder. Archives of General Psychiatry, 62(2), 190–198. https://doi.org/10.1001/archpsyc.62.2.190
- Stein, M. B. (2024). Posttraumatic stress disorder in adults: Treatment overview. UpToDate. https://www.uptodate.com/contents/posttraumatic-stress-disorder-in-adults-treatment-overview
- Bandelow, B., Allgulander, C., Baldwin, D. S., Costa, D. L. da C., Denys, D., Dilbaz, N., & Zohar, J. (2022). World federation of societies of biological psychiatry (WFSBP) guidelines for treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders – version 3. Part II: OCD and PTSD. The World Journal of Biological Psychiatry, 24(2), 118–134. https://doi.org/10.1080/15622975.2022.2086296
- Hsiao, M.-C., & Liu, C.-Y. (2003). Effective open-label treatment of premenstrual dysphoric disorder with venlafaxine. Psychiatry and Clinical Neurosciences, 57(3), 317–321. https://doi.org/10.1046/j.1440-1819.2003.01123.x
- Cohen, L. S., Soares, C. N., Lyster, A., Cassano, P., Brandes, M., & Leblanc, G. A. (2004). Efficacy and Tolerability of Premenstrual Use of Venlafaxine (Flexible Dose) in the Treatment of Premenstrual Dysphoric Disorder. Journal of Clinical Psychopharmacology, 24(5), 540–543. https://doi.org/10.1097/01.jcp.0000138767.53976.10
- Kelly, K., Posternak, M., & Jonathan, E. A. (2008). Toward achieving optimal response: Understanding and managing antidepressant side effects. Dialogues in Clinical Neuroscience, 10(4), 409–418. https://doi.org/10.31887/DCNS.2008.10.4/kkelly
- Winter, J., Curtis, K., Hu, B., & Clayton, A. H. (2022). Sexual dysfunction with major depressive disorder and antidepressant treatments: Impact, assessment, and management. Expert Opinion on Drug Safety, 21(7), 913–930. https://doi.org/10.1080/14740338.2022.2049753
- Goldberg, J. F., & Ernst, C. L. (2022). Managing the side effects of psychotropic medications (2nd ed.). American Psychiatric Association Publishing
- Thompson, S., Compton, L., Chen, J.-L., & Fang, M.-L. (2021). Pharmacologic treatment of antidepressant-induced excessive sweating: A systematic review. Archives of Clinical Psychiatry (São Paulo), 48, 57–65. https://doi.org/10.15761/0101-60830000000279
- Calvi, A., Fischetti, I., Verzicco, I., Belvederi Murri, M., Zanetidou, S., Volpi, R., Coghi, P., Tedeschi, S., Amore, M., & Cabassi, A. (2021). Antidepressant Drugs Effects on Blood Pressure. Frontiers in Cardiovascular Medicine, 8, 704281. https://doi.org/10.3389/fcvm.2021.704281
- Gheysens, T., Van Den Eede, F., & De Picker, L. (2024). The risk of antidepressant-induced hyponatremia: A meta-analysis of antidepressant classes and compounds. European Psychiatry, 67(1), e20. https://doi.org/10.1192/j.eurpsy.2024.11
- Drugs and Lactation Database (LactMed). (2006). Venlafaxine. In Drugs and Lactation Database (LactMed). National Institute of Child Health and Human Development. http://www.ncbi.nlm.nih.gov/books/NBK501192/
- Uguz, F. (2021). A New Safety Scoring System for the Use of Psychotropic Drugs During Lactation. American Journal of Therapeutics, 28(1), e118–e126. https://doi.org/10.1097/MJT.0000000000000909
- Larsen, E. R., Damkier, P., Pedersen, L. H., Fenger-Gron, J., Mikkelsen, R. L., Nielsen, R. E., Linde, V. J., Knudsen, H. E. D., Skaarup, L., & Videbech, P. (2015). Use of psychotropic drugs during pregnancy and breast-feeding. Acta Psychiatrica Scandinavica, 132(S445), 1–28. https://doi.org/10.1111/acps.12479



