Slides and Transcript
Slide 1 of 25
I want to turn now to the role of two new drugs in the treatment of postpartum depression – brexanolone and zuranolone.
Slide 2 of 25
The antidepressants which we've talked about in the previous sections can of course be used for both postpartum depression and postpartum OCD although at higher doses in OCD but brexanolone and zuranolone have been studied so far only in postpartum depression. So what are these drugs? They are forms of the same drug. They are proprietary formulations of allopregnanolone. Allopregnanolone is a neuroactive steroid that's a positive allosteric modulator at the GABA-A receptor.
References:
- Deligiannidis, K. M., Kroll-Desrosiers, A. R., Mo, S., Nguyen, H. P., Svenson, A., Jaitly, N., Hall, J. E., Barton, B. A., Rothschild, A. J., & Shaffer, S. A. (2016). Peripartum neuroactive steroid and γ-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology, 70, 98-107.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 3 of 25
It's a metabolite of progesterone so it rises substantially across pregnancy and then falls abruptly in the postpartum as does progesterone.
References:
- Deligiannidis, K. M., Kroll-Desrosiers, A. R., Mo, S., Nguyen, H. P., Svenson, A., Jaitly, N., Hall, J. E., Barton, B. A., Rothschild, A. J., & Shaffer, S. A. (2016). Peripartum neuroactive steroid and γ-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology, 70, 98-107
Slide 4 of 25
So what's the rationale? Why do we think that these drugs might work in the postpartum? Well, we know that GABAergic hypofunction has been associated with postpartum depression and as I mentioned both brexanolone and zuranolone are positive allosteric modulators of the GABA-A receptor. We therefore think that by increasing GABAergic function these drugs may help to treat postpartum depression.
References:
- Deligiannidis, K. M., Kroll-Desrosiers, A. R., Mo, S., Nguyen, H. P., Svenson, A., Jaitly, N., Hall, J. E., Barton, B. A., Rothschild, A. J., & Shaffer, S. A. (2016). Peripartum neuroactive steroid and γ-aminobutyric acid profiles in women at-risk for postpartum depression. Psychoneuroendocrinology, 70, 98-107.
- Maguire, J., & Mody, I. (2009). Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinology, 34(Suppl 1), S84-S90. https://doi.org/10.1016/j.psyneuen.2009.06.019
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 5 of 25
So how do they work and what's the evidence? Well, we don't really understand the full mechanism of action but the hypothesis is that by returning allopregnanolone concentrations to those of the third trimester we will somehow ameliorate symptoms.
References:
- Reddy, D. S., Mbilinyi, R. H., & Estes, E. (2023). Preclinical and clinical pharmacology of brexanolone (allopregnanolone) for postpartum depression: a landmark journey from concept to clinic in neurosteroid replacement therapy. Psychopharmacology, 240(9), 1841-1863. https://doi.org/10.1007/s00213-023-06427-2
Slide 6 of 25
And how does it work? Brexanolone is an IV infusion. It was first approved by the FDA in 2019 and remember that these drugs were approved only for postpartum depression. They do not have an indication for major depression. And that is an indication that the biology of postpartum depression may be distinct from that of major depressive disorder.
References:
- Kanes, S. J., Colquhoun, H., Doherty, J., Raines, S., Hoffmann, E., Rubinow, D. R., & Meltzer-Brody, S. (2017). Open-label, proof-of-concept study of brexanolone in the treatment of severe postpartum depression. Human Psychopharmacology: Clinical and Experimental, 32(2), e2576. https://doi.org/10.1002/hup.2576
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 7 of 25
Brexanolone was approved first in 2019 and it's a 60-hour IV infusion. There's an initial 12-hour titration period where you begin at 25% of the full dose and work up to 100% of the full dose over that 12-hour period.
References:
- Kanes, S. J., Colquhoun, H., Doherty, J., Raines, S., Hoffmann, E., Rubinow, D. R., & Meltzer-Brody, S. (2017). Open-label, proof-of-concept study of brexanolone in the treatment of severe postpartum depression. Human Psychopharmacology: Clinical and Experimental, 32(2), e2576. https://doi.org/10.1002/hup.2576
Slide 8 of 25
Then for 36-hours, we will maintain that infusion at that maintenance dose and then we have a 12-hour taper down. The taper is meant to prevent withdrawal symptoms from a GABA active agent.
References:
- Kanes, S. J., Colquhoun, H., Doherty, J., Raines, S., Hoffmann, E., Rubinow, D. R., & Meltzer-Brody, S. (2017). Open-label, proof-of-concept study of brexanolone in the treatment of severe postpartum depression. Human Psychopharmacology: Clinical and Experimental, 32(2), e2576. https://doi.org/10.1002/hup.2576
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 9 of 25
During the infusion, women are monitored for two things. First, excessive sedation. One has to immediately stop the infusion at any sign of excessive sedation and then you can resume it after the symptoms resolve at the same or reduced dose as clinically appropriate.
References:
- Patterson, R., Balan, I., Morrow, A. L., Holman, A. J., Brinton, R. D., Barth, V. N., Kanes, S. J., & Meltzer-Brody, S. (2024). Novel neurosteroid therapeutics for post-partum depression: Perspectives on clinical trials, program development, active research, and future directions. Neuropsychopharmacology, 49, 67–72. https://doi.org/10.1038/s41386-023-01721-1
Slide 10 of 25
Women are also monitored for hypoxia and it's important to immediately stop the infusion if pulse oximetry indicates hypoxia and not resume the therapy.
References:
- Patterson, R., Balan, I., Morrow, A. L., Holman, A. J., Brinton, R. D., Barth, V. N., Kanes, S. J., & Meltzer-Brody, S. (2024). Novel neurosteroid therapeutics for post-partum depression: Perspectives on clinical trials, program development, active research, and future directions. Neuropsychopharmacology, 49, 67–72. https://doi.org/10.1038/s41386-023-01721-1
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 11 of 25
What this means is that these two reasons for continuous monitoring mean that people taking brexanolone have to be monitored continuously either by telemetry or by a one-to-one sitter in the hospital and that makes this a somewhat onerous drug to administer.
References:
- Patterson, R., Balan, I., Morrow, A. L., Holman, A. J., Brinton, R. D., Barth, V. N., Kanes, S. J., & Meltzer-Brody, S. (2024). Novel neurosteroid therapeutics for post-partum depression: Perspectives on clinical trials, program development, active research, and future directions. Neuropsychopharmacology, 49, 67–72. https://doi.org/10.1038/s41386-023-01721-1
Slide 12 of 25
What do the data show us about whether this drug works? Well, the initial trial was an open-label study with patients who had a prior postpartum depression, some of whom were taking concomitant psychotropic medications.
References:
- Kanes, S. J., Colquhoun, H., Doherty, J., Raines, S., Hoffmann, E., Rubinow, D. R., & Meltzer-Brody, S. (2017). Open-label, proof-of-concept study of brexanolone in the treatment of severe postpartum depression. Human Psychopharmacology: Clinical and Experimental, 32(2), e2576. https://doi.org/10.1002/hup.2576
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 13 of 25
And in that initial open-label study, there was a dramatic response. Within just a few days, people responded extremely quickly. So then the company moved on to a placebo-controlled phase 2 style that had 11 patients on placebo and 10 patients taking brexanolone.
References:
- Patterson, R., Balan, I., Morrow, A. L., Holman, A. J., Brinton, R. D., Barth, V. N., Kanes, S. J., & Meltzer-Brody, S. (2024). Novel neurosteroid therapeutics for post-partum depression: Perspectives on clinical trials, program development, active research, and future directions. Neuropsychopharmacology, 49, 67–72. https://doi.org/10.1038/s41386-023-01721-1
Slide 14 of 25
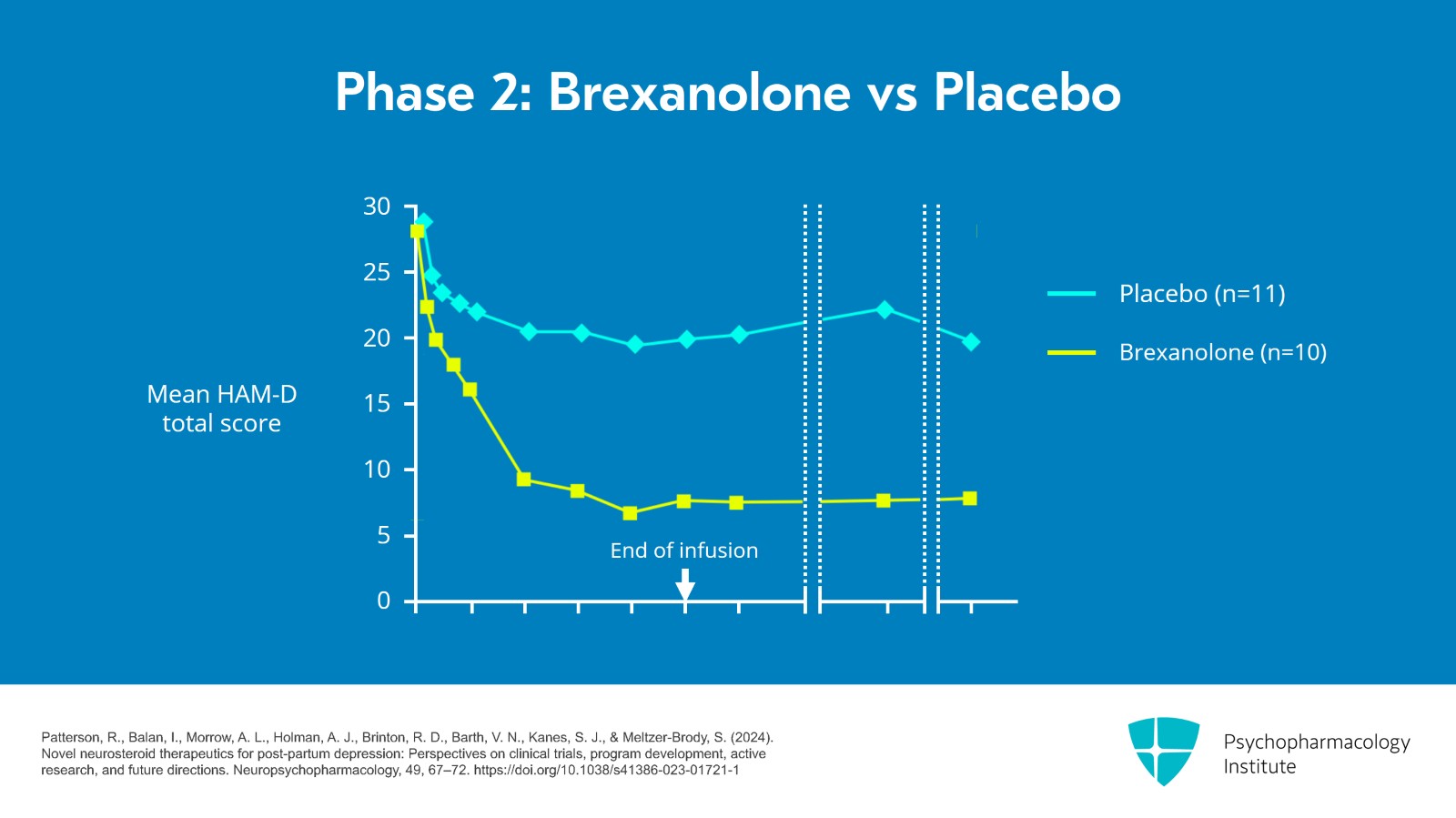
Each patient received a single continuous intravenous infusion of the masked study drug. And at 60 hours, the mean reduction in the HAM-D total score from baseline was 21 points in the brexanolone group compared with 8.8 points in the placebo group.
References:
- Patterson, R., Balan, I., Morrow, A. L., Holman, A. J., Brinton, R. D., Barth, V. N., Kanes, S. J., & Meltzer-Brody, S. (2024). Novel neurosteroid therapeutics for post-partum depression: Perspectives on clinical trials, program development, active research, and future directions. Neuropsychopharmacology, 49, 67–72. https://doi.org/10.1038/s41386-023-01721-1
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 15 of 25
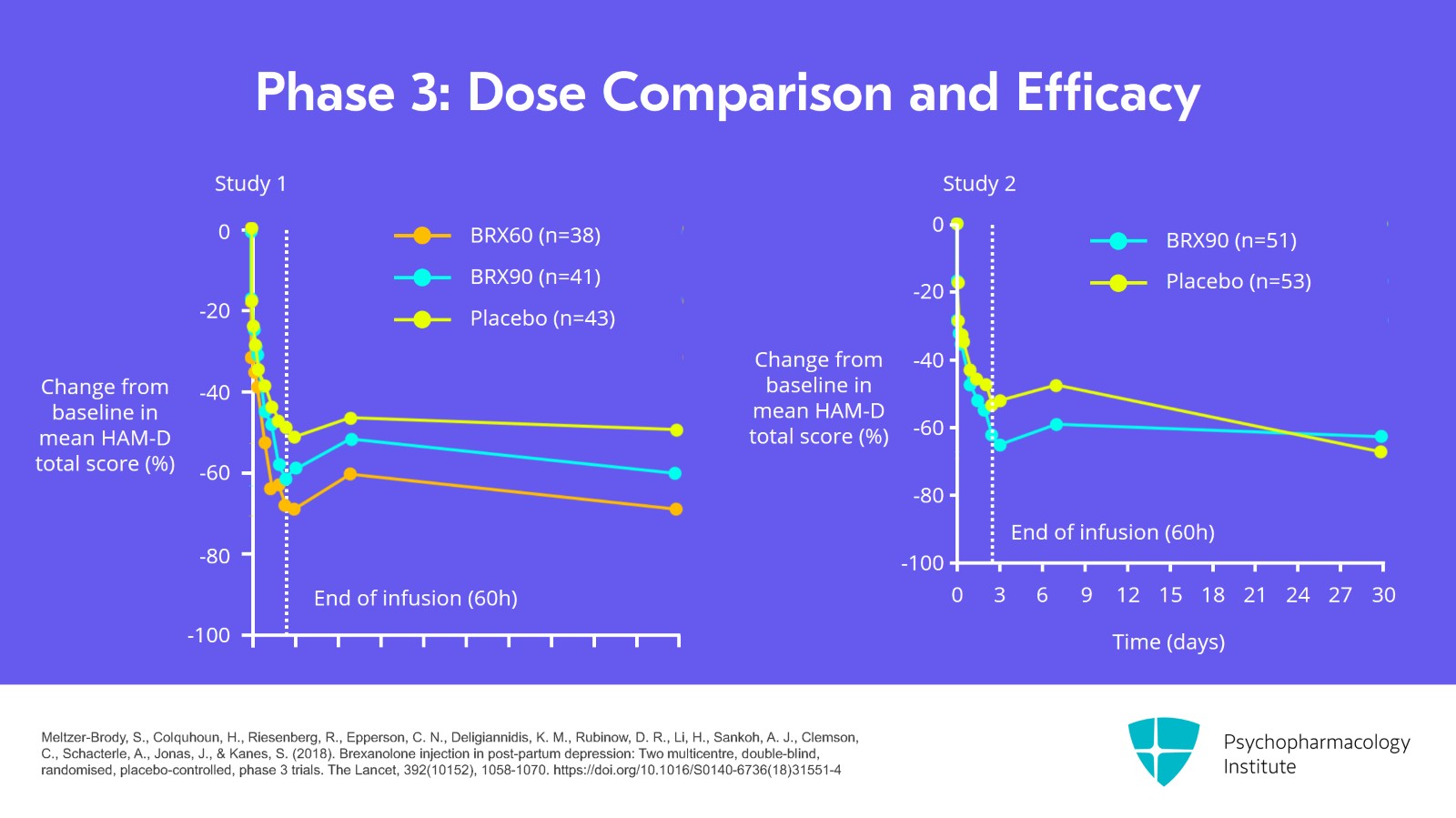
For phase 3 studies, the company conducted two double-blind, randomized, placebo-controlled phase 3 trials. One of them compared 60 mg/kg/hr versus placebo and versus 90 mg/kg/hr and the second study just compared 90 mg to the placebo.
References:
- Meltzer-Brody, S., Colquhoun, H., Riesenberg, R., Epperson, C. N., Deligiannidis, K. M., Rubinow, D. R., Li, H., Sankoh, A. J., Clemson, C., Schacterle, A., Jonas, J., & Kanes, S. (2018). Brexanolone injection in post-partum depression: Two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. The Lancet, 392(10152), 1058-1070. https://doi.org/10.1016/S0140-6736(18)31551-415
Slide 16 of 25
In a combined analysis of the two studies that integrated, the study found that 90 mg was the optimal dose compared to placebo and it showed a significant reduction from baseline in HAM-D scores for that combined group.
References:
- Meltzer-Brody, S., Colquhoun, H., Riesenberg, R., Epperson, C. N., Deligiannidis, K. M., Rubinow, D. R., Li, H., Sankoh, A. J., Clemson, C., Schacterle, A., Jonas, J., & Kanes, S. (2018). Brexanolone injection in post-partum depression: Two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. The Lancet, 392(10152), 1058-1070. https://doi.org/10.1016/S0140-6736(18)31551-416
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 17 of 25
As a result, the FDA approved this for IV infusion and the drug has been available since 2019 but with limited uptake due to the difficulty of administration and that need for continuous monitoring.
References:
- Patterson, R., Balan, I., Morrow, A. L., Holman, A. J., Brinton, R. D., Barth, V. N., Kanes, S. J., & Meltzer-Brody, S. (2024). Novel neurosteroid therapeutics for post-partum depression: Perspectives on clinical trials, program development, active research, and future directions. Neuropsychopharmacology, 49, 67–72. https://doi.org/10.1038/s41386-023-01721-117
Slide 18 of 25
Because of that, the company announced that they filed an application with the FDA drug for an orally bioavailable form of that same drug. This is called zuranolone which was approved by the FDA just a year ago and has been available clinically for patients since about December 2023.
References:
- Patterson, R., Balan, I., Morrow, A. L., Holman, A. J., Brinton, R. D., Barth, V. N., Kanes, S. J., & Meltzer-Brody, S. (2024). Novel neurosteroid therapeutics for post-partum depression: Perspectives on clinical trials, program development, active research, and future directions. Neuropsychopharmacology, 49, 67–72. https://doi.org/10.1038/s41386-023-01721-1
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 19 of 25
This drug also met its primary endpoint which was a separation from placebo by day 15 of the treatment. The drug was studied and randomization to one to one, placebo versus zuranolone and was studied at several different dose regimens.
References:
- Deligiannidis, K. M., Meltzer-Brody, S., Gunduz-Bruce, H., Doherty, J., Jonas, J., Li, S., Sankoh, A. J., Silber, C., Campbell, A. D., Werneburg, B., Eriksson, H., Clemson, C., Epperson, C. N., & Kanes, S. (2021). Effect of zuranolone vs placebo in postpartum depression: A randomized clinical trial. JAMA Psychiatry, 78(9), 951–959. https://doi.org/10.1001/jamapsychiatry.2021.1559
Slide 20 of 25
As I mentioned, it became available in December 2023 and it has a high price tag but as of this writing most insurances including Medicaid are covering the drug in full. Prescriptions have to be sent to a mail order pharmacy which can be frustratingly slow.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 21 of 25
And we have to remember that the medication must be taken at night with a meal of at least 400 calories. The most prominent side effect is sedation with some patients reporting significant sedation at night and an indication that patients are not allowed to drive or use heavy machinery for 12 hours after taking the drug.
References:
- Deligiannidis, K. M., Meltzer-Brody, S., Gunduz-Bruce, H., Doherty, J., Jonas, J., Li, S., Sankoh, A. J., Silber, C., Campbell, A. D., Werneburg, B., Eriksson, H., Clemson, C., Epperson, C. N., & Kanes, S. (2021). Effect of zuranolone vs placebo in postpartum depression: A randomized clinical trial. JAMA Psychiatry, 78(9), 951–959. https://doi.org/10.1001/jamapsychiatry.2021.1559
Slide 22 of 25
The drug is taken for two weeks and then stopped. And remission of symptoms usually occurs within three days. We don't yet have any information about long-term outcomes for zuranolone but it may be a promising treatment as many women are attracted to the idea of taking a drug that they can take just for two weeks and then stop.
References:
- Deligiannidis, K. M., Meltzer-Brody, S., Gunduz-Bruce, H., Doherty, J., Jonas, J., Li, S., Sankoh, A. J., Silber, C., Campbell, A. D., Werneburg, B., Eriksson, H., Clemson, C., Epperson, C. N., & Kanes, S. (2021). Effect of zuranolone vs placebo in postpartum depression: A randomized clinical trial. JAMA Psychiatry, 78(9), 951–959. https://doi.org/10.1001/jamapsychiatry.2021.1559
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 23 of 25
So let's review some key points for this section. In the past five years, we've seen two new drugs approved for postpartum depression. These are the first drugs ever to be approved by the FDA specifically for postpartum depression and they may indicate that there is indeed a different mechanism of action for postpartum depression than for depression at other time points. The mechanism of action of these drugs is based on a lot of basic science research that implicated the role of neuroactive steroids that are metabolites of progesterone in the etiology of postpartum depression.
Slide 24 of 25
Both drugs are synthetic versions of allopregnanolone, a metabolite of progesterone that's an allosteric modulator at the GABA-A receptor. Brexanolone is an IV formulation. Zuranolone is an oral formulation that's brand new and has been on the market for only a few months. We don't yet have much data about long-term effectiveness or about compatibility with breastfeeding with either drug but they may prove to be an important new tool in the armamentarium in postpartum depression.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.