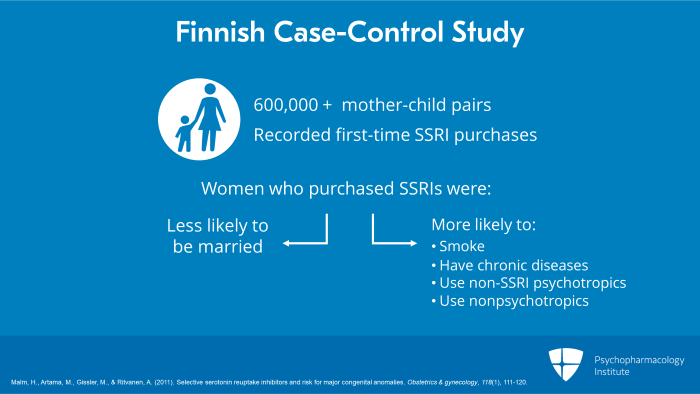
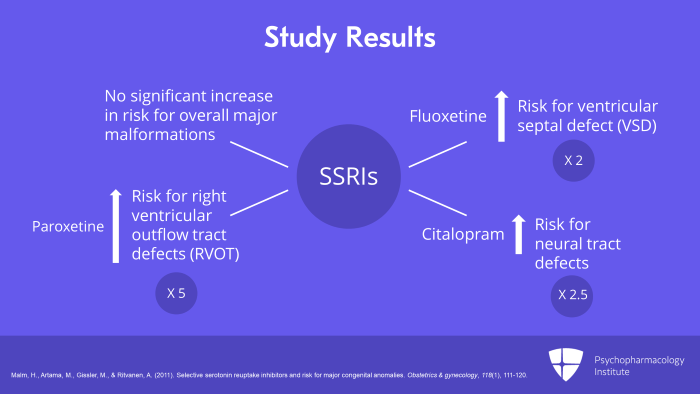
And what were the results? SSRIs as a group and individually, there was no significant increase in risk for overall major malformations. However, fluoxetine was found to increase the risk for ventricular septal defect (VSD) twofold. And paroxetine increased the risk for right ventricular outflow tract defects (RVOT) fivefold. And citalopram increased neural tract defects 2-1/2-fold. So those are concerning
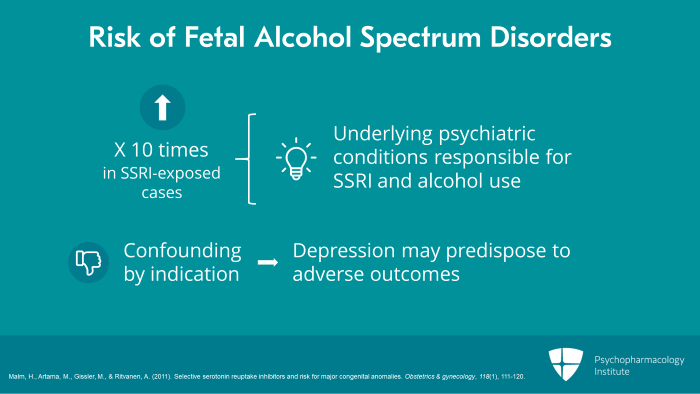
However, notice also that the risk of fetal alcohol spectrum disorders was 10 times higher in SSRI-exposed cases. So this suggests underlying psychiatric conditions which were responsible for both SSRI and alcohol use, fetal alcohol spectrum disorders. The study suggests confounding by indication with depression may have predisposed to adverse outcomes rather than SSRIs themselves.
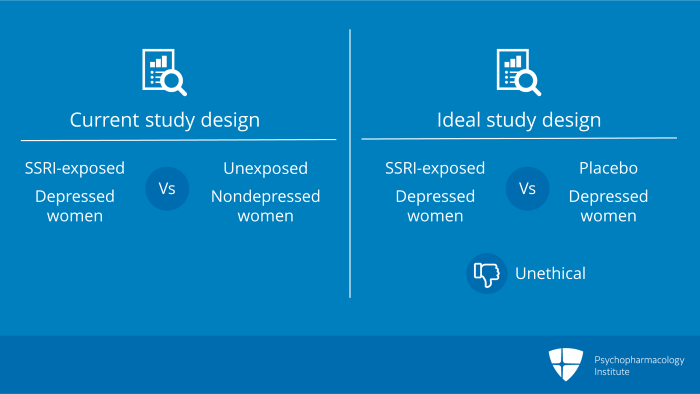
Notice, if you will, the study design included SSRI-exposed depressed women who were compared with unexposed non-depressed women. What was the study that should’ve been done? Well, what we needed were randomized controlled data where depressed women were randomized to SSRI or placebo but this can never be done. It is unethical in pregnancy to withhold medication from disabled depressed women. This is the problem with case-control, database linked studies.
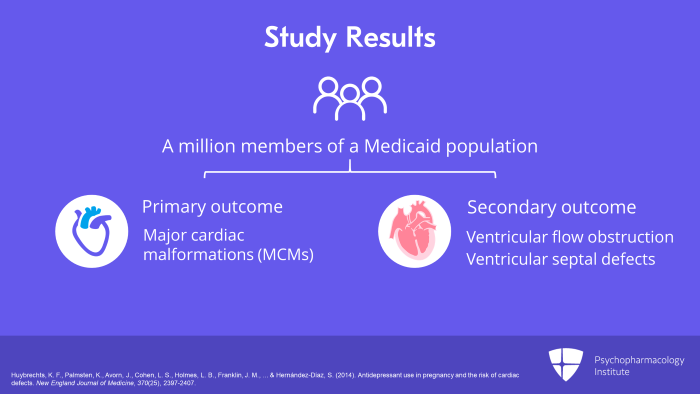
Now, moving on to another study, a study by Huybrechts, et al. in 2014. This was a population-based cohort study done by the Agency for Healthcare Research and Quality, the AHRQ, and the NIH, the National Institute for Health. And it looked at almost a million members of a Medicaid population. And what were the outcomes that were looked at? The primary outcome was major cardiac malformations (MCMs) among first trimester antidepressant-exposed infants versus non-exposed infants. And the secondary outcome was honing in and looking at types of cardiac defects particularly ventricular flow obstruction and ventricular septal defects.
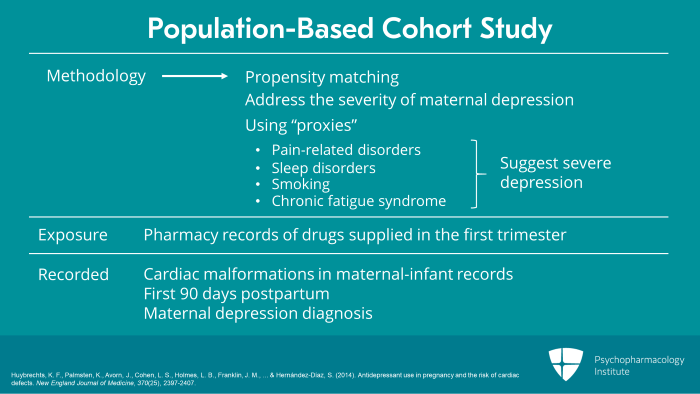
What they used in terms of methodology was propensity matching. First, they looked at pharmacy records where exposure was defined by drug supplied in the first trimester. Cardiac malformations were recorded in maternal-infant records up to the first 90 days postpartum. They also recorded maternal depression diagnosis. And when I talked about propensity matching, what they did was to address the severity of maternal depression. They used what we call proxies that is pain-related disorders, sleep disorders, smoking, chronic fatigue syndrome, all of which when this occurs in women who are depressed suggest that these women have significant severe depression. It addresses the likelihood that the depression for which these patients were treated was severe.
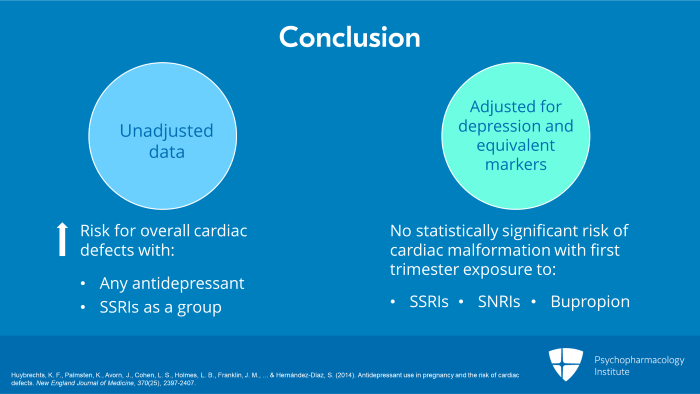
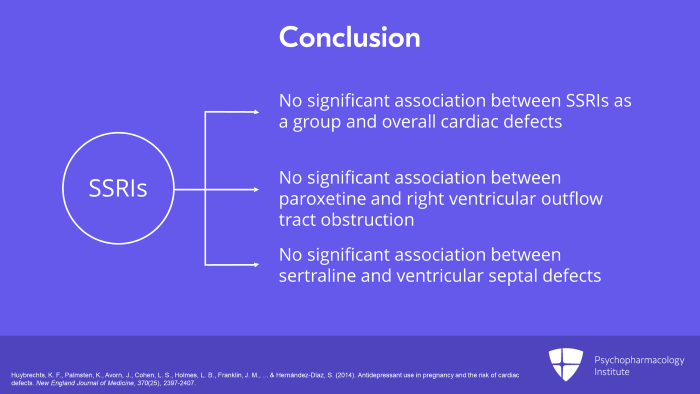
And what was the conclusion? The conclusion was that when you looked at the unadjusted data, there was an increased risk for overall cardiac defects with any antidepressants and with SSRIs as a group. But when adjusted for the diagnosis of depression and depressive equivalent markers, there was no statistically significant risk of any cardiac malformation with first trimester exposure to any antidepressants, not to SSRIs, not to SNRIs and not to bupropion.
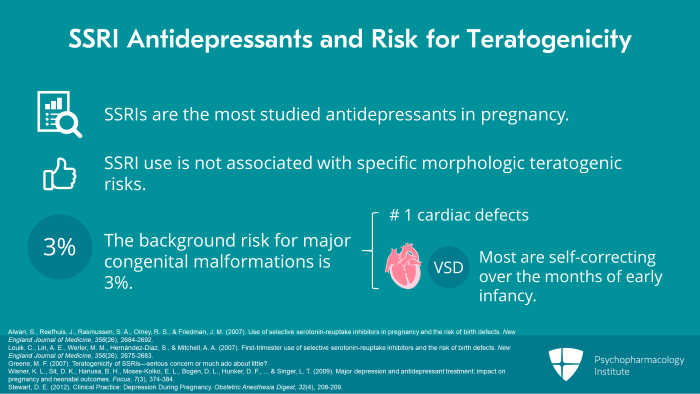
So when we look at SSRI antidepressants and the risk for teratogenicity, a general statement that we can make is that SSRIs in fact are the most studied antidepressants in pregnancy. We have well over 33,000 reported published exposures. Overall, SSRI use is not associated with specific morphologic teratogenic risks and we have to always remember that the background risk for major congenital malformations is 3%. And I should add the most common of these congenital malformations is cardiac defects. And of those, the most common are VSD or ventricular septal defects, many of which correct themselves over the early infancy months.

So let’s end with some key points here for this section. First of all, overall, SSRI use has not been associated with specific morphologic teratogenic risks. Number two, remember maternal stress and depression during pregnancy are associated with serious maternal illness, poor health behaviors and increased risk for postpartum illness. And number three, discontinuation of antidepressants that keep a woman healthy during pregnancy is associated with a relapse of depression and incurs negative consequences for mother, for baby and her family.
References
- Malm, H., Artama, M., Gissler, M., & Ritvanen, A. (2011). Selective serotonin reuptake inhibitors and risk for major congenital anomalies . Obstetrics & gynecology, 118(1), 111-120.
- Huybrechts, K. F., Palmsten, K., Avorn, J., Cohen, L. S., Holmes, L. B., Franklin, J. M., … & Hernández-Díaz, S. (2014). Antidepressant use in pregnancy and the risk of cardiac defects . New England Journal of Medicine, 370(25), 2397-2407.
- Alwan, S., Reefhuis, J., Rasmussen, S. A., Olney, R. S., & Friedman, J. M. (2007). Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects . New England Journal of Medicine, 356(26), 2684-2692.
- Louik, C., Lin, A. E., Werler, M. M., Hernández-Díaz, S., & Mitchell, A. A. (2007). First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects . New England Journal of Medicine, 356(26), 2675-2683.
- Greene, M. F. (2007). Teratogenicity of SSRIs—serious concern or much ado about little? .
- Wisner, K. L., Sit, D. K., Hanusa, B. H., Moses-Kolko, E. L., Bogen, D. L., Hunker, D. F., … & Singer, L. T. (2009). Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes . Focus, 7(3), 374-384.
- Stewart, D. E. (2012). Clinical Practice: Depression During Pregnancy. Obstetric Anesthesia Digest , 32(4), 208-209.



