Slides and Transcript
Slide 1 of 9
In this section, I’ll discuss antiepileptic drugs and the use in bipolar disorder during pregnancy.
Slide 2 of 9
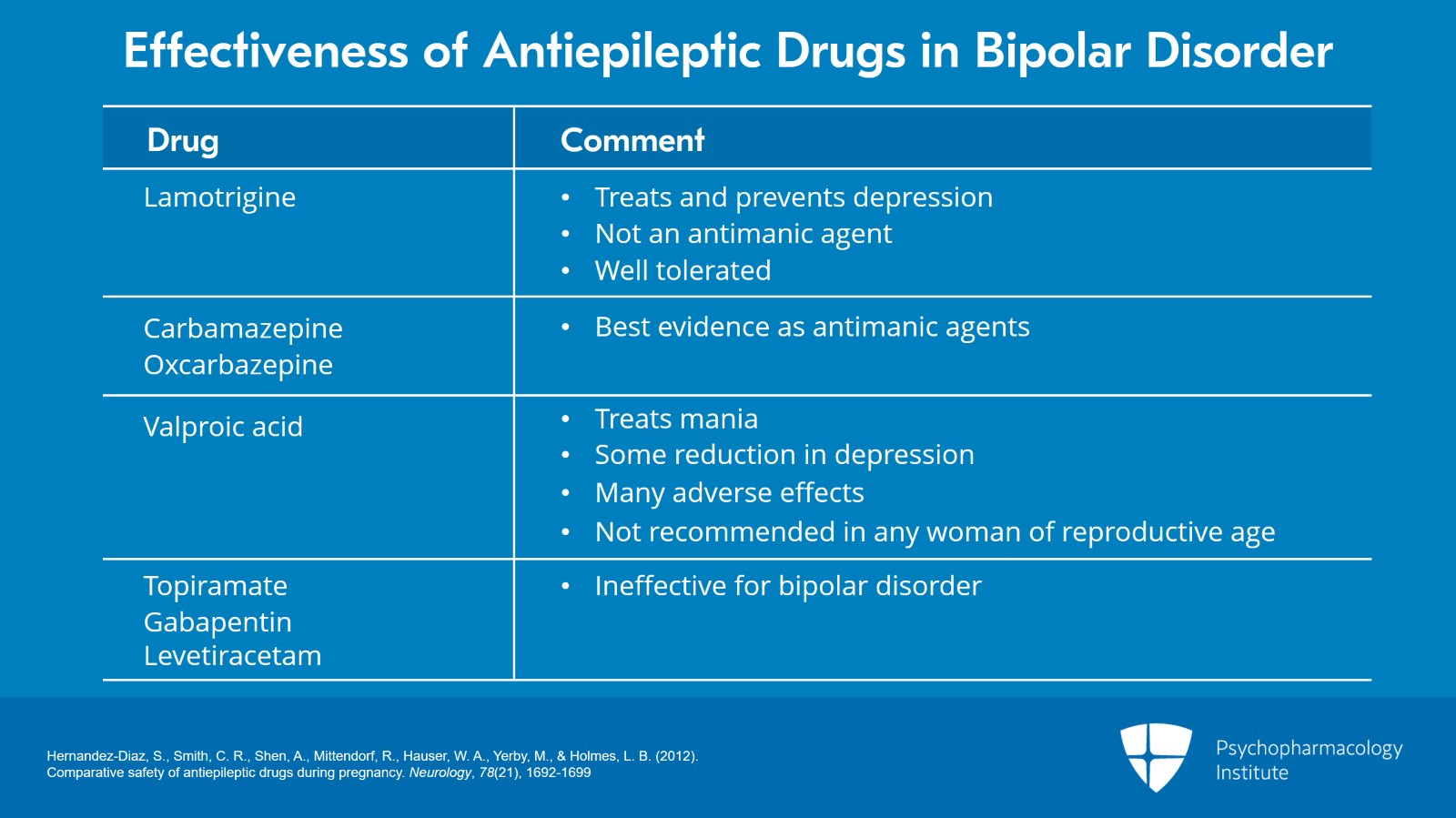
And again, let me begin with an overview of the effectiveness of antiseizure medications in bipolar disorder.
Lamotrigine treats depression, has indication for prevention of depression in bipolar disorder. However, it’s not an antimanic agent. While it can help maintain mood stability over time, it’s not directly an antimanic medication. It is well tolerated.
Carbamazepine and oxcarbazepine both have the best evidence as antimanic agents.
Valproic acid or valproate treats mania, appears to have some reduction in depression. It does have many adverse effects and needs levels to be monitored. And I’m just going to start now in telling you it’s not recommended in any reproductive-age woman.
I’m also going to give a brief mention of medications that are ineffective for bipolar disorder that are antiseizure medications such as topiramate, gabapentin, levetiracetam. We don’t want to be using these for mood stability as they have negative studies.
So in looking at antiepileptic drugs in pregnancy, we have vastly different risks. In contrast to typical versus atypical antipsychotics, you know, there’s a number of similarities in risks when used in pregnancy, not so with the antiseizure meds.
References:
- Hernandez-Diaz, S., Smith, C. R., Shen, A., Mittendorf, R., Hauser, W. A., Yerby, M., & Holmes, L. B. (2012). Comparative safety of antiepileptic drugs during pregnancy. Neurology, 78(21), 1692-1699
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 3 of 9
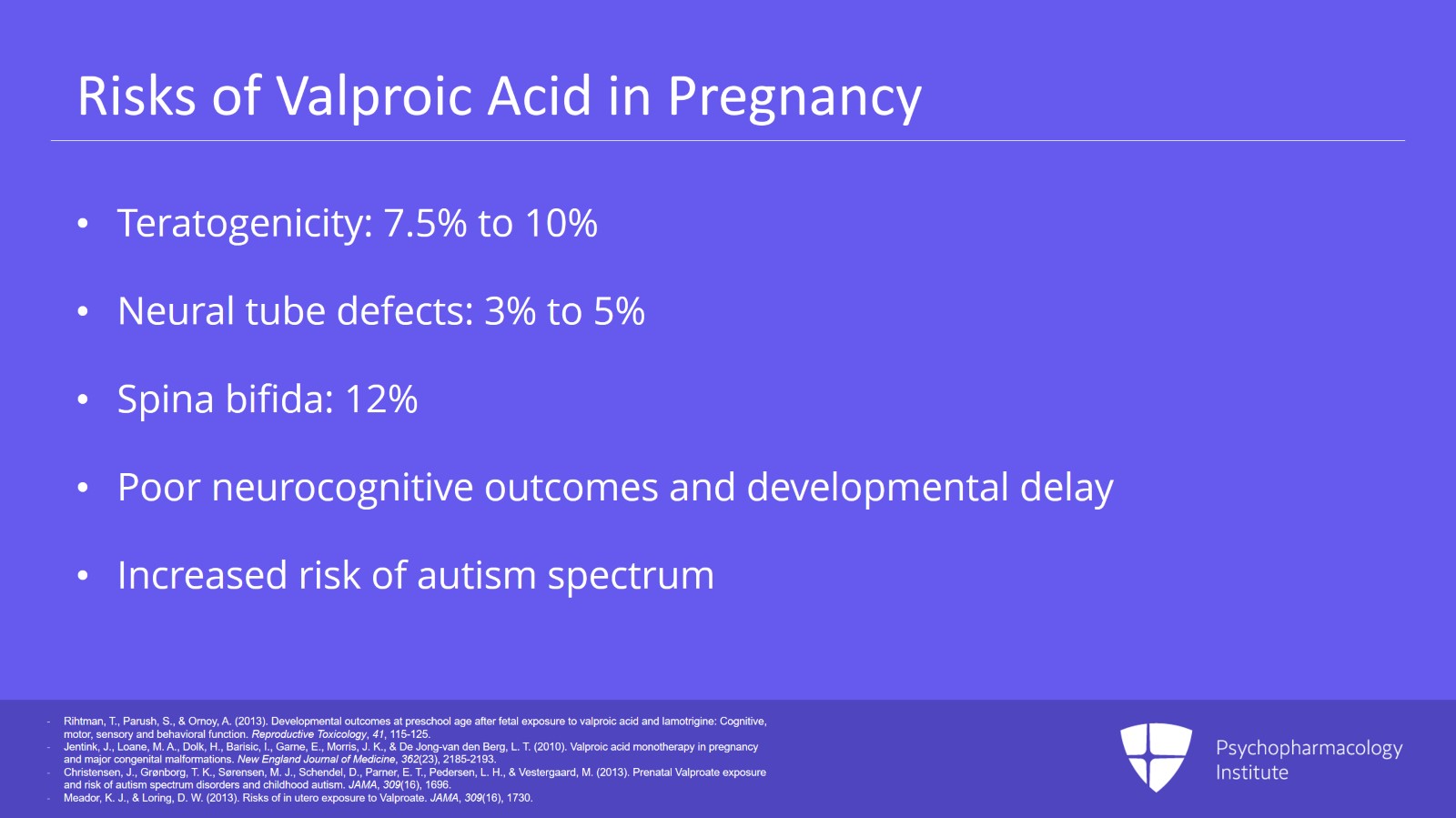
I’m going to start with valproic acid, valproate, divalproex, all the same thing. It has a known increased teratogenicity risk with use in the first trimester. This is up to 7.5% to 10%.
Neural tube defects alone have a range of 3% to 5% and this is the general population risk for any major malformation, that 3% to 5%. But with divalproex, that’s just neural tube defects alone.
Well, spina bifida is the most notoriously associated risk with valproic acid use in pregnancy. We see an increased odds ratio of major malformations from many organ systems. So a New England Journal of Medicine study found an increased risk of spina bifida of 12.7% odds ratio, atrial septal, cleft palate, hypospadias, polydactyly, craniosynostosis. We’re hitting many organ systems here.
Not only that, valproic acid has poor neurocognitive outcomes as well. Development is delayed in children who are exposed to valproic acid in utero. IQ tends to be 10 points lower than expected.
There’s an increased risk of autism spectrum in these children.
In fact, valproate has been banned for use in pregnancy in France. The concerns have been so great. Okay. So not a medication you want to be using in pregnancy, if at all possible to avoid.
References:
- Rihtman, T., Parush, S., & Ornoy, A. (2013). Developmental outcomes at preschool age after fetal exposure to valproic acid and lamotrigine: Cognitive, motor, sensory and behavioral function. Reproductive Toxicology, 41, 115-125.
- Jentink, J., Loane, M. A., Dolk, H., Barisic, I., Garne, E., Morris, J. K., & De Jong-van den Berg, L. T. (2010). Valproic acid monotherapy in pregnancy and major congenital malformations. New England Journal of Medicine, 362(23), 2185-2193.
- Christensen, J., Grønborg, T. K., Sørensen, M. J., Schendel, D., Parner, E. T., Pedersen, L. H., & Vestergaard, M. (2013). Prenatal Valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA, 309(16), 1696.
- Meador, K. J., & Loring, D. W. (2013). Risks of in utero exposure to Valproate. JAMA, 309(16), 1730.
Slide 4 of 9
Carbamazepine and oxcarbazepine I see rarely used clinically these days. There’s notably a lack of reliable data on the use of these in pregnancy but here’s a sampling of what we do know.
Pregnancy course is poorly documented in these medications. Birth outcomes have included low birth weight though that hasn’t necessarily been consistent.
There have been reports of higher teratogenicity up to two times the population average with concerns about neural tube, cardiac, urinary, craniofacial risk increase versus a report of no increased risk in major malformations with the use of oxcarbazepine.
Neurocognitively, questionable outcomes. Reports of developmental delay versus no effect on IQ. I think this picture is still being sorted out. If either of these two are to be used in pregnancy, emphasizing in the discussion that there’s a lot that’s unknown on their effects on fetal development and course.
References:
- Diav-Citrin, O., Shechtman, S., Arnon, J., & Ornoy, A. (2001). Is carbamazepine teratogenic?: A prospective controlled study of 210 pregnancies. Neurology, 57(2), 321-324.
- Weston, J., Bromley, R., Jackson, C. F., Adab, N., Clayton-Smith, J., Greenhalgh, J., Hounsome, J., McKay, A. J., Tudur Smith, C., & Marson, A. G. (2016). Monotherapy treatment of epilepsy in pregnancy: Congenital malformation outcomes in the child. Cochrane Database of Systematic Reviews.
- Andrade, C. (2018). Major congenital malformations associated with exposure to Antiepileptic drugs during pregnancy. The Journal of Clinical Psychiatry, 79(4).
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 5 of 9
Moving on to lamotrigine. In contrast, this is actually fairly well studied and characterized in pregnancy. These antiseizure medications, the data are primarily on women with seizure disorders. So it’s not directly women with bipolar disorder but the effects on pregnancy course, birth outcomes, teratogenicity we don’t anticipate being particularly different in women with bipolar versus seizure disorder.
But given that, lamotrigine use in pregnancy in the course, we’re not seeing an increased risk of miscarriage or stillbirth or increased risk of preterm delivery.
Lamotrigine use effect on birth outcomes, we’re not seeing an increased risk of small for gestational age. We’re not seeing reports of adaptation syndrome, at least not compared to symptoms above the general population. And teratogenicity, lamotrigine use in pregnancy has a teratogenicity rate within the range of the general population at about 3%.
A note on that, cleft palate in the early literature was reported as potentially an increased risk of which of the major malformations. But this has not been replicated. So overall, we’re not finding an increased risk of teratogenicity at this point.
Neurocognitive is reassuring. These children are hitting their milestones on time and their IQ is as expected despite their exposure to lamotrigine in utero. So it’s been reassuring to know.
References:
- Pariente, G., Leibson, T., Shulman, T., Adams-Webber, T., Barzilay, E., & Nulman, I. (2017). Pregnancy outcomes following in utero exposure to Lamotrigine: A systematic review and meta-analysis. CNS Drugs, 31(6), 439-450.
- Andrade, C. (2018). Major congenital malformations associated with exposure to Antiepileptic drugs during pregnancy. The Journal of Clinical Psychiatry, 79(4).
- Kong, L., Zhou, T., Wang, B., Gao, Z., & Wang, C. (2017). The risks associated with the use of lamotrigine during pregnancy. International Journal of Psychiatry in Clinical Practice, 22(1), 2-5.
Slide 6 of 9
Okay. I’m going to transition here on how to prescribe antiseizure medication for bipolar disorder in pregnancy particularly with lamotrigine because this can be complicated more so than atypicals.
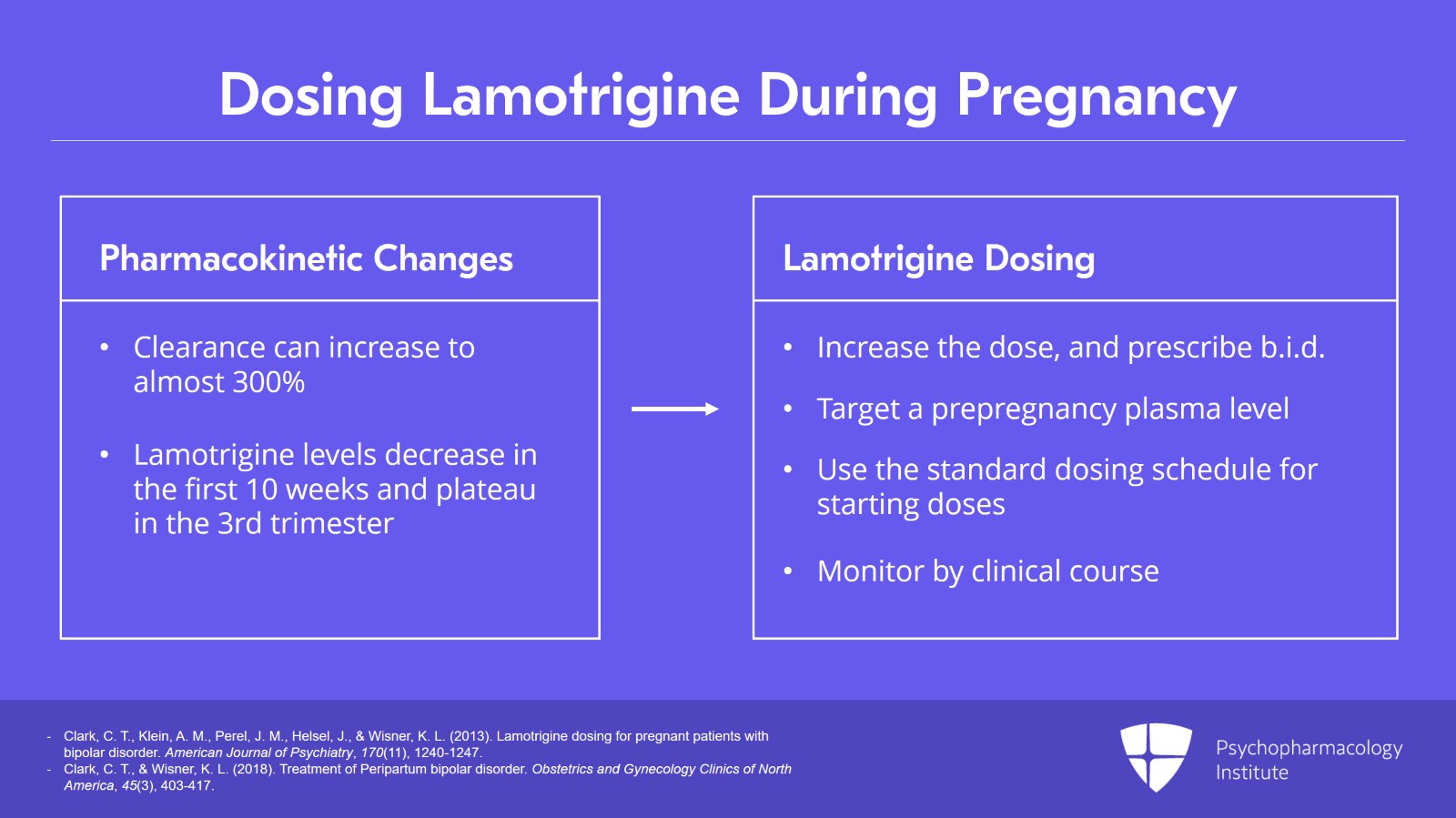
So lamotrigine pharmacokinetics in pregnancy have been fairly well characterized. Clearance can increase to almost 300%, three times that of a non-pregnant woman. So levels start decreasing in the first 10 weeks and they plateau in the third trimester.
There is a need to increase dose to keep the level steady and commonly this can be two to three times the pre-pregnancy dose. B.i.d. dosing is recommended like with lithium with the idea to help keep the plasma levels more stable.
And ideally, the prescriber will target a pre-pregnancy plasma level that was taken when the patient was euthymic.
So we don’t typically follow lamotrigine levels in pregnancy and this is where thinking in the preconception planning of getting that level when the woman is doing well on the lamotrigine to target if or when she becomes pregnant.
However, if you’re starting lamotrigine in pregnancy, we have no data to recommend different starting doses or starting uptitration rates than when not pregnant. So you’re going to use your standard dosing schedule. And without a pre-pregnancy level when this happens, our goal is to have the woman maintain euthymia. Lamotrigine dosing is best monitored by clinical course. If the woman starts to show early signs of depression, then an increase in dosing may very well be warranted.
References:
- Clark, C. T., Klein, A. M., Perel, J. M., Helsel, J., & Wisner, K. L. (2013). Lamotrigine dosing for pregnant patients with bipolar disorder. American Journal of Psychiatry, 170(11), 1240-1247.
- Clark, C. T., & Wisner, K. L. (2018). Treatment of Peripartum bipolar disorder. Obstetrics and Gynecology Clinics of North America, 45(3), 403-417.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 7 of 9
So let’s take a moment to return to Jess, our clinical vignette.
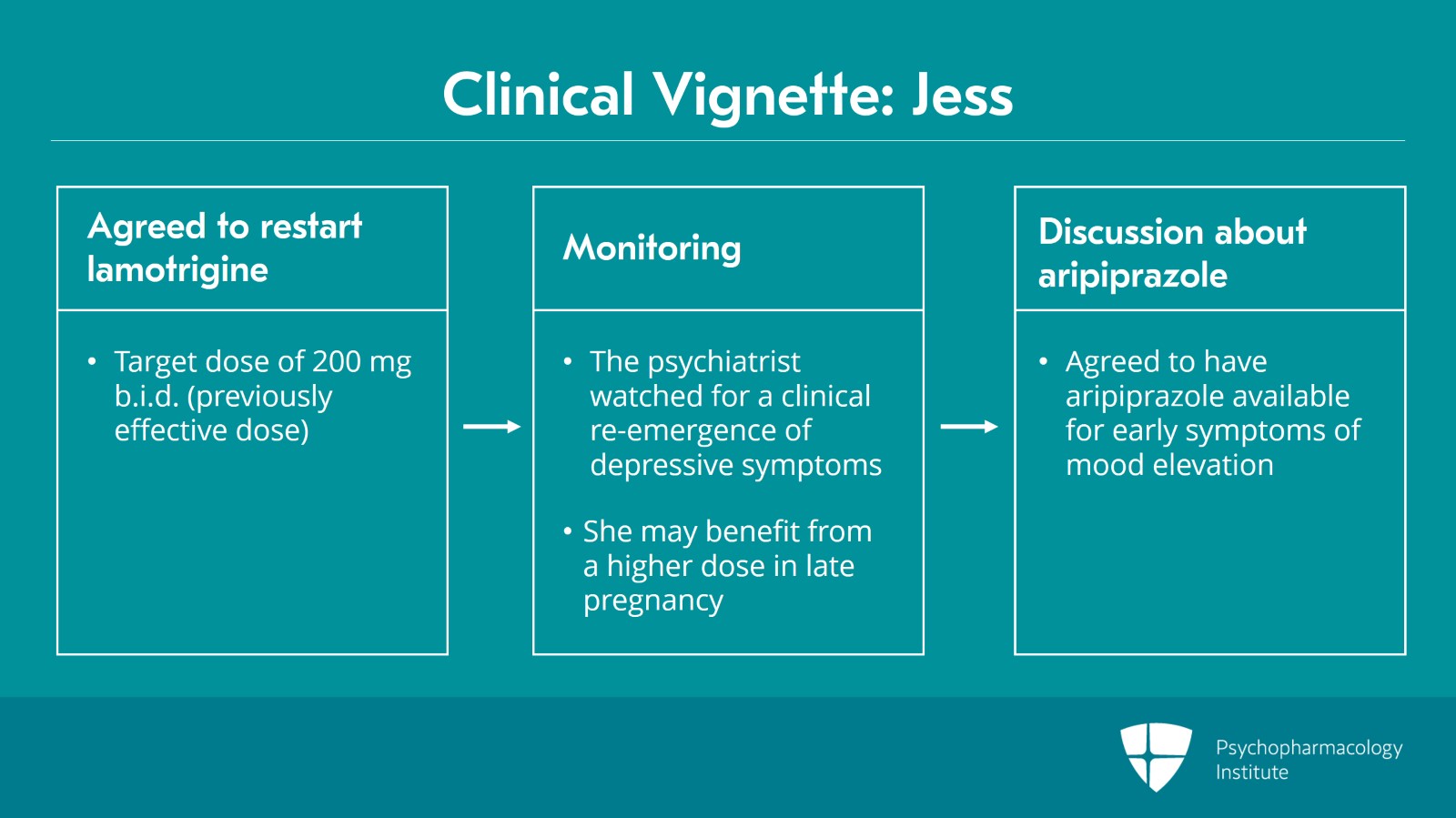
She agreed to be restarted on lamotrigine to treat her bipolar depression and the plan was to target the previously effective dose of 200 mg b.i.d. using the standard uptitration dosing guidelines.
As there was no lamotrigine level obtained prior to pregnancy when the patient was euthymic, the psychiatrist watched for clinical reemergence of bipolar depression symptoms knowing that lamotrigine levels decline substantially later in pregnancy and the patient may very well benefit from a higher dose range than she was using prior to pregnancy where that may be in the PDR for non-pregnant women.
Upon discussion with the patient of the risks and benefits of restarting aripiprazole for mood elevation in pregnancy, the patient and provider decided to have the medication available immediately or p.r.n. for early symptoms of mood elevation rather than a daily use for prevention.
They came to this decision given patient had good insight into the first symptoms of hypomania, she had good motivation and intent to treat the mood elevations and she had had over a year since her most recent mood elevation so a period without a mood elevation.
Slide 8 of 9
So key points from this section include avoiding valproic acid or valproate in reproductive-age women because of the high risk of teratogenicity.
Current data on lamotrigine use in pregnancy and outcome are reassuring.
And then being aware of the need to adjust or increase the dose of lamotrigine through pregnancy.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.