Slides and Transcript
Slide 1 of 14
In this video, you're going to learn about the genetic tests that really matter in practice.
Slide 2 of 14
Earlier, I compared pharmacokinetic genes to drug interactions, and that comparison should've raised some eyebrows for you. I mean, how many times do you get alerts about drug interactions in your electronic medical record? And do you delve into all of them or suffer from alert fatigue? So why should we give these pharmacogenetic alerts any more weight? The answer depends on the medication. It's not just the gene that matters but the gene-drug interaction.
References:
- Phansalkar, S., van der Sijs, H., Tucker, A. D., Desai, A. A., Bell, D. S., Teich, J. M., Middleton, B., & Bates, D. W. (2013). Drug-drug interactions that should be non-interruptive in order to reduce alert fatigue in electronic health records. Journal of the American Medical Informatics Association, 20(3), 489-493. https://doi.org/10.1136/amiajnl-2012-001089
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 3 of 14
Most psych meds have a wide therapeutic window and a wide margin of safety, so we don't check blood levels or worry too much about drug interactions with them. Many are metabolized by multiple enzymes in the liver. So if one of them slows down, another can take over. Two groups, the Clinical Pharmacogenetics Implementation Consortium and the US Food and Drug Administration or FDA, keep an updated list of drug-gene interactions that matter. To make their list, there has to be clinical evidence that the drug-gene interaction could significantly compromise the medication's safety or efficacy. When it comes to safety, the risk these groups are most concerned about is cardiac.
References:
- Li, M.-Y., Peng, L.-M., & Chen, X.-P. (2022). Pharmacogenomics in drug-induced cardiotoxicity: Current status and the future. Frontiers in Cardiovascular Medicine, 9. https://doi.org/10.3389/fcvm.2022.966261
- Ehmann, F., Caneva, L., Prasad, K., Paulmichl, M., Maliepaard, M., Llerena, A., Ingelman-Sundberg, M., & Papaluca-Amati, M. (2015). Pharmacogenomic information in drug labels: European Medicines Agency perspective. The Pharmacogenomics Journal, 15(3), 201-210. https://doi.org/10.1038/tpj.2014.86
Slide 4 of 14
When drugs that prolong the QTc interval reach high levels, it can trigger a potentially fatal arrhythmia called torsades de pointes. In the United States, the FDA requires genetic testing before going to higher doses of pimozide and recommends testing for the medications thioridazine, citalopram and the tardive dyskinesia medications deutetrabenazine and valbenazine. All of these are for the QTc interaction.
References:
- Li, M.-Y., Peng, L.-M., & Chen, X.-P. (2022). Pharmacogenomics in drug-induced cardiotoxicity: Current status and the future. Frontiers in Cardiovascular Medicine, 9. https://doi.org/10.3389/fcvm.2022.966261
- Bousman, C. A., Zierhut, H., & Müller, D. J. (2019). Navigating the Labyrinth of Pharmacogenetic Testing: A Guide to Test Selection. Clinical Pharmacology & Therapeutics, 106(2), 309-312. https://doi.org/10.1002/cpt.1432
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 5 of 14
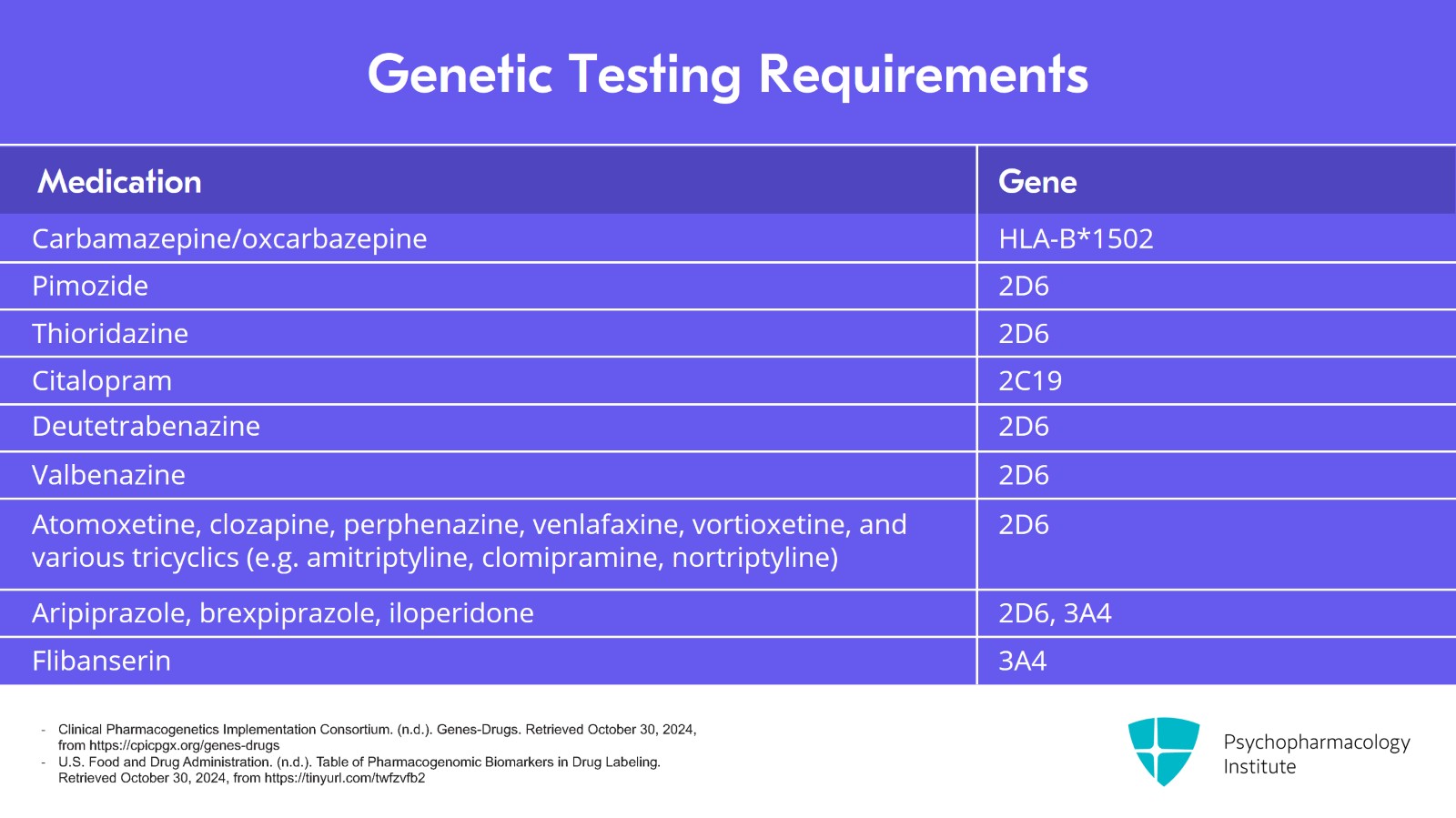
The FDA is not requiring a full genetic panel here. They only want you to check the enzyme that metabolizes the drug to avoid high levels and potential arrhythmias. The table on the screen lists psychiatric drugs for which these groups recommend genetic testing before prescribing them. You'll notice that three pharmacokinetic tests cover nearly all of the requirements. Those are CYP2D6, 3A4 and C19. And I agree. These are the most useful of the genetic tests. But there is one more that is very useful and in fact required by the FDA, and it's not a pharmacokinetic gene. It is the HLA typing for carbamazepine. Keep this list of actionable drug-gene interactions, say, if a patient calls with unusually severe side effects on one of these medications. If the drug is on this list, they might be a poor metabolizer and genetic testing is warranted.
References:
- Clinical Pharmacogenetics Implementation Consortium. (n.d.). Genes-Drugs. Retrieved October 30, 2024, from https://cpicpgx.org/genes-drugs
- U.S. Food and Drug Administration. (n.d.). Table of Pharmacogenomic Biomarkers in Drug Labeling. Retrieved October 30, 2024, from https://tinyurl.com/twfzvfb2
Slide 6 of 14
Let's look at how that plays out with the ADHD medication atomoxetine, Strattera. Atomoxetine causes sedation in about 1 in 20 patients but for those 1 in 20 the problem can be quite severe, so severe that atomoxetine ranks near the top of medications with an antidepressant structure that triggered reports of sedation to the FDA surveillance system based on a study that classified atomoxetine as an antidepressant which it was originally designed as. How did a rare side effect make it to the top of that list? Well, atomoxetine levels peaked 10-fold higher in people who are poor CYP2D6 metabolizers. So with this medication, when it rains, it pours.
References:
- Eugene, A. R. (2020). Association of sleep among 30 antidepressants: A population-wide adverse drug reaction study, 2004–2019. PeerJ, 8, e8748. https://doi.org/10.7717/peerj.8748
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 7 of 14
Let's pause to recap some of the key points. Just as with most drug-drug interactions, most gene-drug interactions do not make a meaningful difference in practice. Several groups have compiled the gene-drug interactions that you need to be cautious about. The list includes antipsychotics, tricyclic antidepressants, citalopram, tardive dyskinesia medications and atomoxetine.
Slide 8 of 14
Now, let's look at the outlier on that list, a gene for a drug allergy with carbamazepine. One of these genes is not like the others. It's the HLA-B*1502 gene and it predicts whether patients of Asian descent will have a serious rash on carbamazepine and possibly on oxcarbazepine. Patients who are positive for the HLA-B*1502 are 80 times more likely to develop Stevens-Johnson syndrome on carbamazepine and they're 30 times more likely to develop it on oxcarbazepine.
References:
- Tham, K. M., Yek, J. J. L., & Liu, C. W. Y. (2024). Unraveling the genetic link: An umbrella review on HLA-B*15:02 and antiepileptic drug-induced Stevens–Johnson syndrome/toxic epidermal necrolysis. Pharmacogenetics and Genomics, 34(5), 154-165. https://doi.org/10.1097/FPC.0000000000000531
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 9 of 14
But what about lamotrigine which can also cause Stevens-Johnson syndrome? Here, the risk is much smaller. They're only twice as likely to develop that rash with lamotrigine. So it's not recommended for testing on lamotrigine as it doesn't change the odds very much. And because this gene is prevalent in Asian populations, the FDA requires it before prescribing carbamazepine to a patient of Asian descent. And you cannot prescribe it if the result is positive. It's just too risky.
References:
- Tham, K. M., Yek, J. J. L., & Liu, C. W. Y. (2024). Unraveling the genetic link: An umbrella review on HLA-B*15:02 and antiepileptic drug-induced Stevens–Johnson syndrome/toxic epidermal necrolysis. Pharmacogenetics and Genomics, 34(5), 154-165. https://doi.org/10.1097/FPC.0000000000000531
Slide 10 of 14
For oxcarbazepine, the testing is recommended by some medical groups but not by the FDA, again, and it's only recommended in Asian populations. For lamotrigine, no guidelines recommend the test because a positive result is not going to change your prescribing. Although it doubles the risk of Stevens-Johnson syndrome, that's still within the margin of error for the estimated risk which runs from 1 in 3000 to 1 in 6000.
References:
- Tham, K. M., Yek, J. J. L., & Liu, C. W. Y. (2024). Unraveling the genetic link: An umbrella review on HLA-B*15:02 and antiepileptic drug-induced Stevens–Johnson syndrome/toxic epidermal necrolysis. Pharmacogenetics and Genomics, 34(5), 154-165. https://doi.org/10.1097/FPC.0000000000000531
- Bloom, R., & Amber, K. T. (2017). Identifying the incidence of rash, Stevens-Johnson syndrome and toxic epidermal necrolysis in patients taking lamotrigine: a systematic review of 122 randomized controlled trials. Anais Brasileiros de Dermatologia, 92(1), 139-141. https://doi.org/10.1590/abd1806-4841.2017507010
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 11 of 14
But what does Asian descent really mean? People with the highest rates of this HLA gene are from Hong Kong, Thailand, Malaysia, China, Taiwan and the Philippines. That's where 10% to 15% of the population has this HLA gene. Rates are lower in South Asia, India and north China where they are 2% to 4% and they're lower still in Japan and Korea where they're less than 1%.
References:
- Lim, K. S., Kwan, P., & Tan, C. T. (2008). Association of HLA-B*1502 allele and carbamazepine-induced severe adverse cutaneous drug reaction among Asians, a review. Neurology Asia, 13, 15-21. https://www.neurologyasia.org/articles/20081_015.pdf
Slide 12 of 14
When it comes to recommended and required genes, there's another one to know about that predicts liver failure on valproic acid but this gene called polymerase gamma or POLG is only present in patients with hereditary neurometabolic syndromes like Alpers-Huttenlocher syndrome. The US Food and Drug Administration requires testing for POLG before prescribing valproic acid in those patients.
References:
- Stewart, J. D., Horvath, R., Baruffini, E., Ferrero, I., Bulst, S., Watkins, P. B., Fontana, R. J., Day, C. P., & Chinnery, P. F. (2010). Polymerase γ gene POLG determines the risk of sodium valproate-induced liver toxicity. Hepatology, 52(5), 1791-1796. https://doi.org/10.1002/hep.23891
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 13 of 14
Let's recap the key points of this talk. Among the genes that have a clinically meaningful effect on treatment, only one is a pharmacodynamic gene, HLA-B*1502. This gene predicts a life-threatening allergic reaction to carbamazepine called Stevens-Johnson syndrome. Testing for this gene is required before prescribing carbamazepine in patients of Asian descent because that is the population where the gene is prevalent.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.