Slides and Transcript
Slide 2 of 24
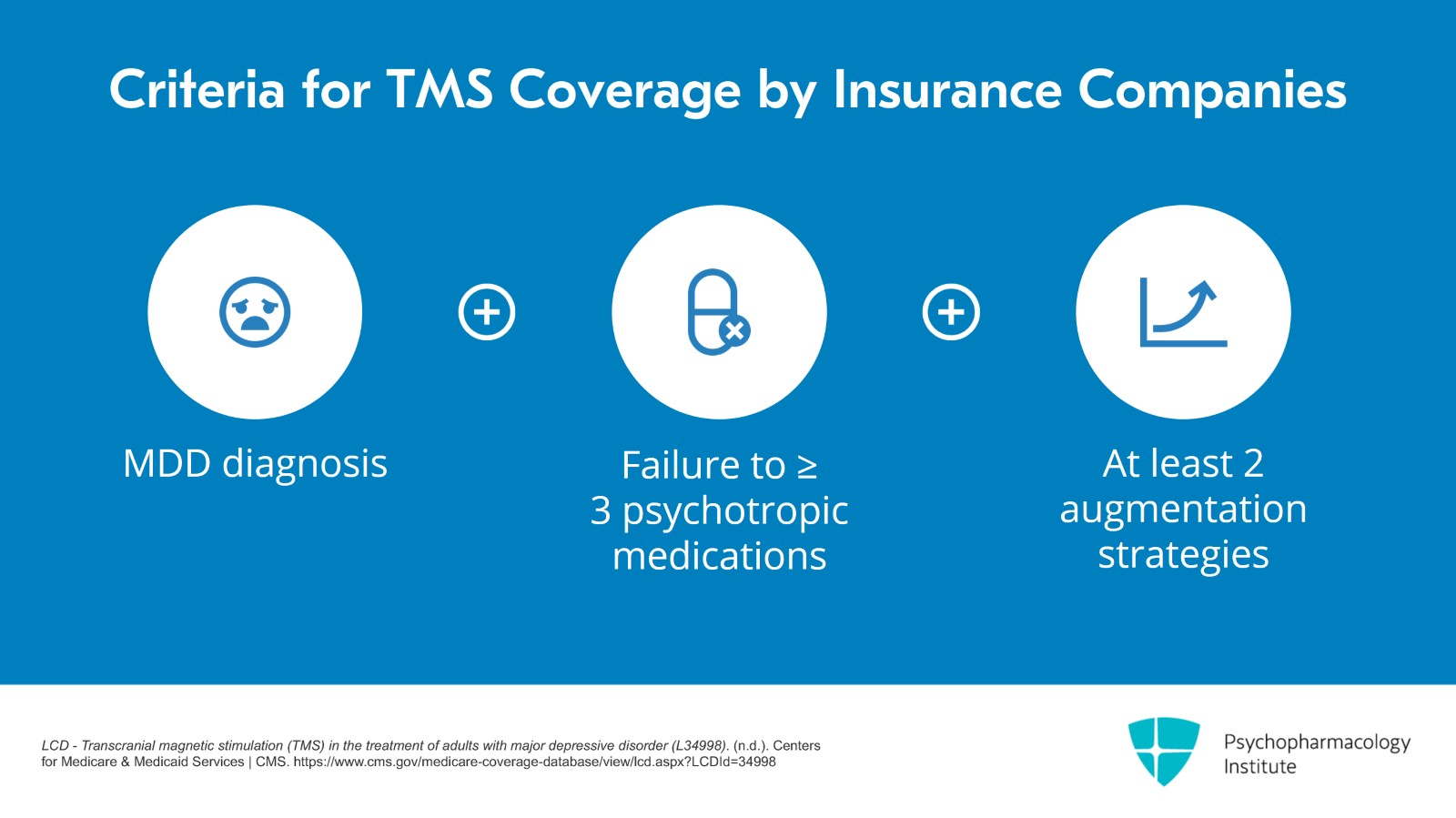
So the official FDA approval is for patients who have not responded to one antidepressant but the practical answer is that it depends on insurance. Most insurance companies will have a medical necessity policy in which the diagnosis has to be major depressive disorder which means that bipolar depression is not covered. They'll want to see that the patient has failed a certain number of psychotropic medications. Usually, it's three to four and at least two of these should be augmentation strategies.
References:
- LCD – Transcranial magnetic stimulation (TMS) in the treatment of adults with major depressive disorder (L34998). (n.d.). Centers for Medicare & Medicaid Services | CMS. https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?LCDId=34998
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 3 of 24
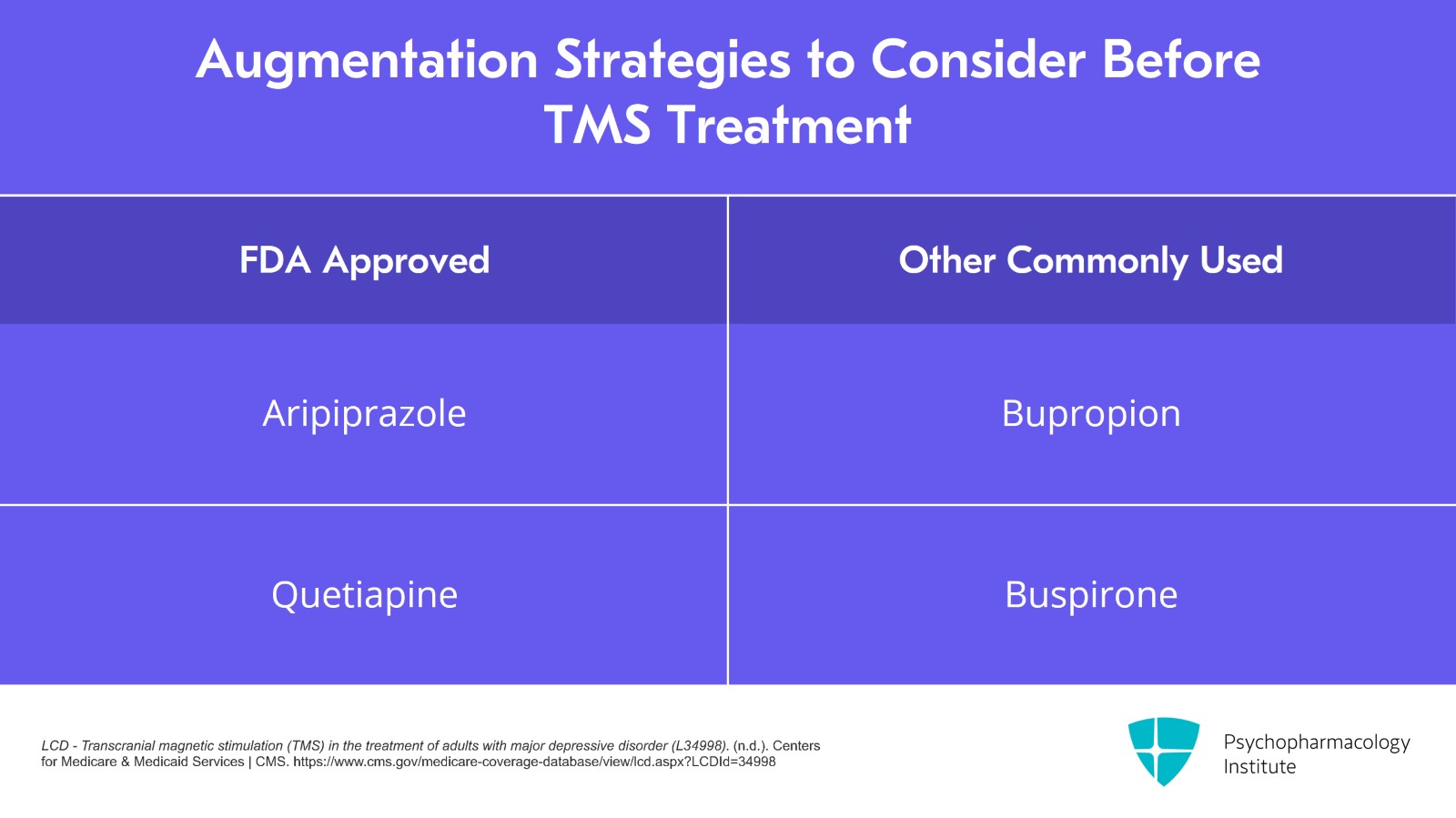
They usually don't define the specific augmentation strategies but certainly FDA-approved ones such as aripiprazole and quetiapine would count and I expect that commonly used ones such as bupropion and buspirone – basically, a second medication added to a first – that would count as well.
References:
- LCD – Transcranial magnetic stimulation (TMS) in the treatment of adults with major depressive disorder (L34998). (n.d.). Centers for Medicare & Medicaid Services | CMS. https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?LCDId=34998
Slide 4 of 24
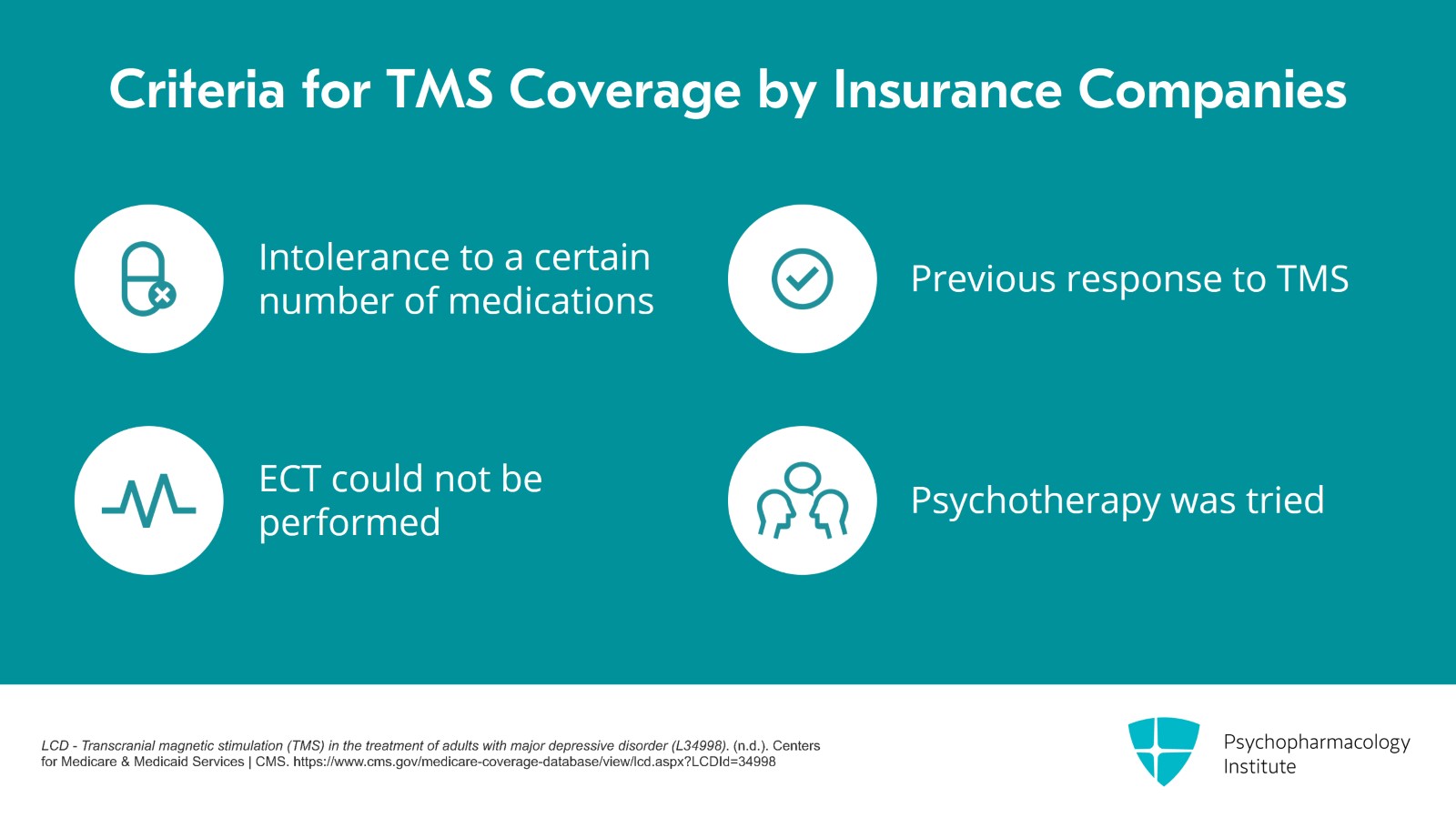
If the patient has not had enough medication trials, intolerance to a certain number of medications could be sufficient. And usually if the patient has had TMS before and had gotten better for at least two to three months or if the patient was recommended for ECT but there were reasons not to do ECT, those are other inclusion criteria. Usually, insurances will want to know that patients have had tried some psychotherapy as well.
References:
- LCD – Transcranial magnetic stimulation (TMS) in the treatment of adults with major depressive disorder (L34998). (n.d.). Centers for Medicare & Medicaid Services | CMS. https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?LCDId=34998
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 5 of 24
To find these criteria, you can do a web search on the insurance company name and transcranial magnetic stimulation and look for the medical necessity policy. To double check, you can call the patient's insurance company to ask or, or have the patient call the insurance company. Sometimes, there's going to be prior authorizations required and you might have to fill out a form listing the patient's previous medication trials.
Slide 6 of 24
One insurance company doesn't cover TBS so you have to perform standard 10 Hz TMS on those covered patients.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 7 of 24
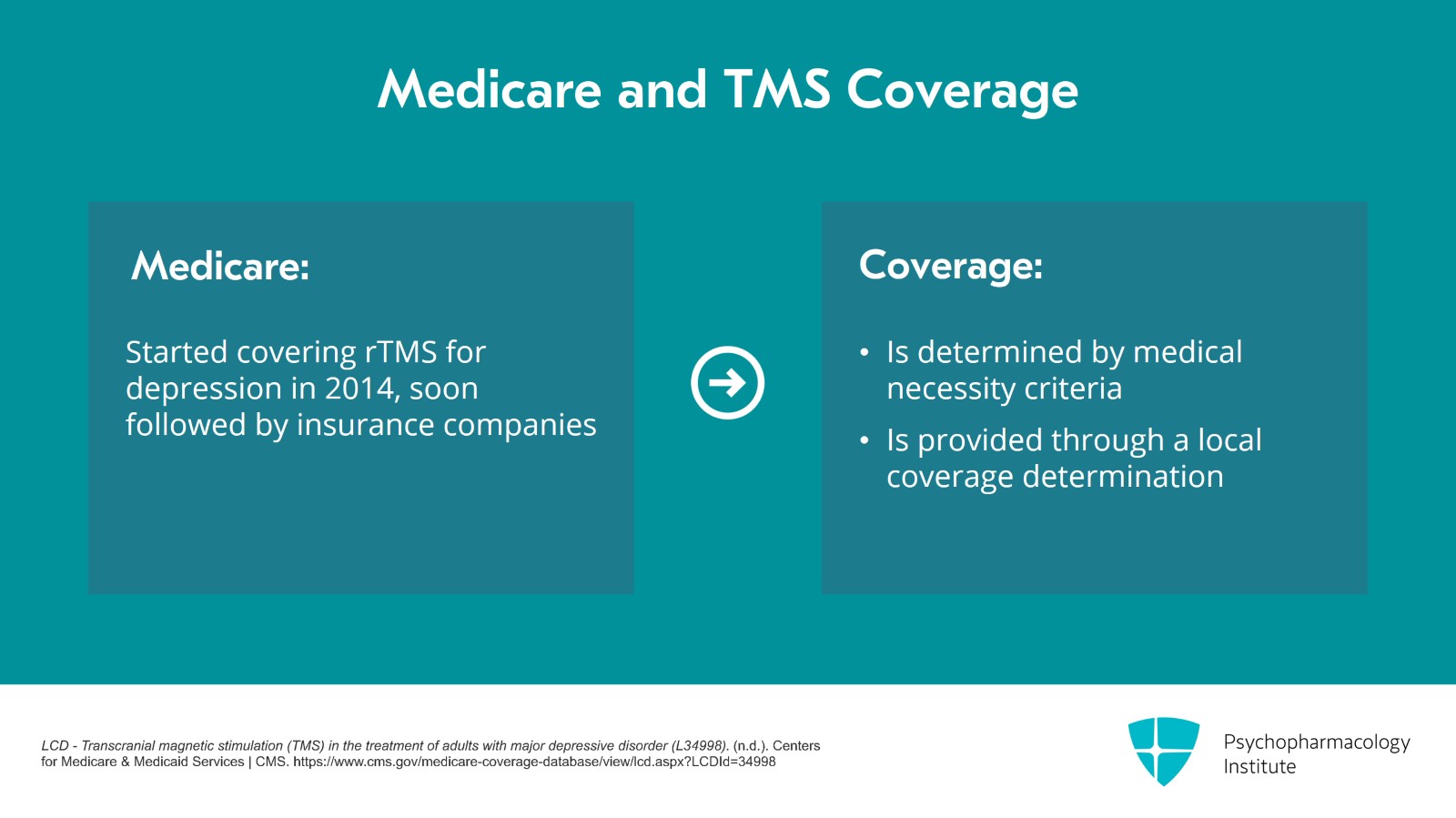
It was Medicare who started covering rTMS for depression in 2014 and shortly after that, commercial insurance companies followed. So for the years between 2008 and 2014, not many patients got treated with TMS for depression because there was no insurance coverage. Medicare also has a medical necessity criteria and it's through a local coverage determination or LCD.
References:
- LCD – Transcranial magnetic stimulation (TMS) in the treatment of adults with major depressive disorder (L34998). (n.d.). Centers for Medicare & Medicaid Services | CMS. https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?LCDId=34998
Slide 8 of 24
There are times when we do not want to do TMS on a patient and that's if the patient has a history of seizures or if there's any concern about metallic objects in the head within 30 cm of the coil including things like vagal nerve stimulator leads. The concern about seizures is that it increases the risk of the patient having a seizure during TMS. The concern about metallic objects is that those could heat up during TMS and cause damage. So for example, if there's cochlear implants or an aneurysm coil, then TMS is not recommended.
References:
- McClintock, S. M., Reti, I. M., Carpenter, L. L., McDonald, W. M., Dubin, M., Taylor, S. F., Cook, I. A., O’Reardon, J., Husain, M. M., Wall, C., Krystal, A. D., Sampson, S. M., Morales, O., Nelson, B. G., Latoussakis, V., George, M. S., & Lisanby, S. H. (2018). Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. The Journal of Clinical Psychiatry, 79(1), 35-48.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 9 of 24
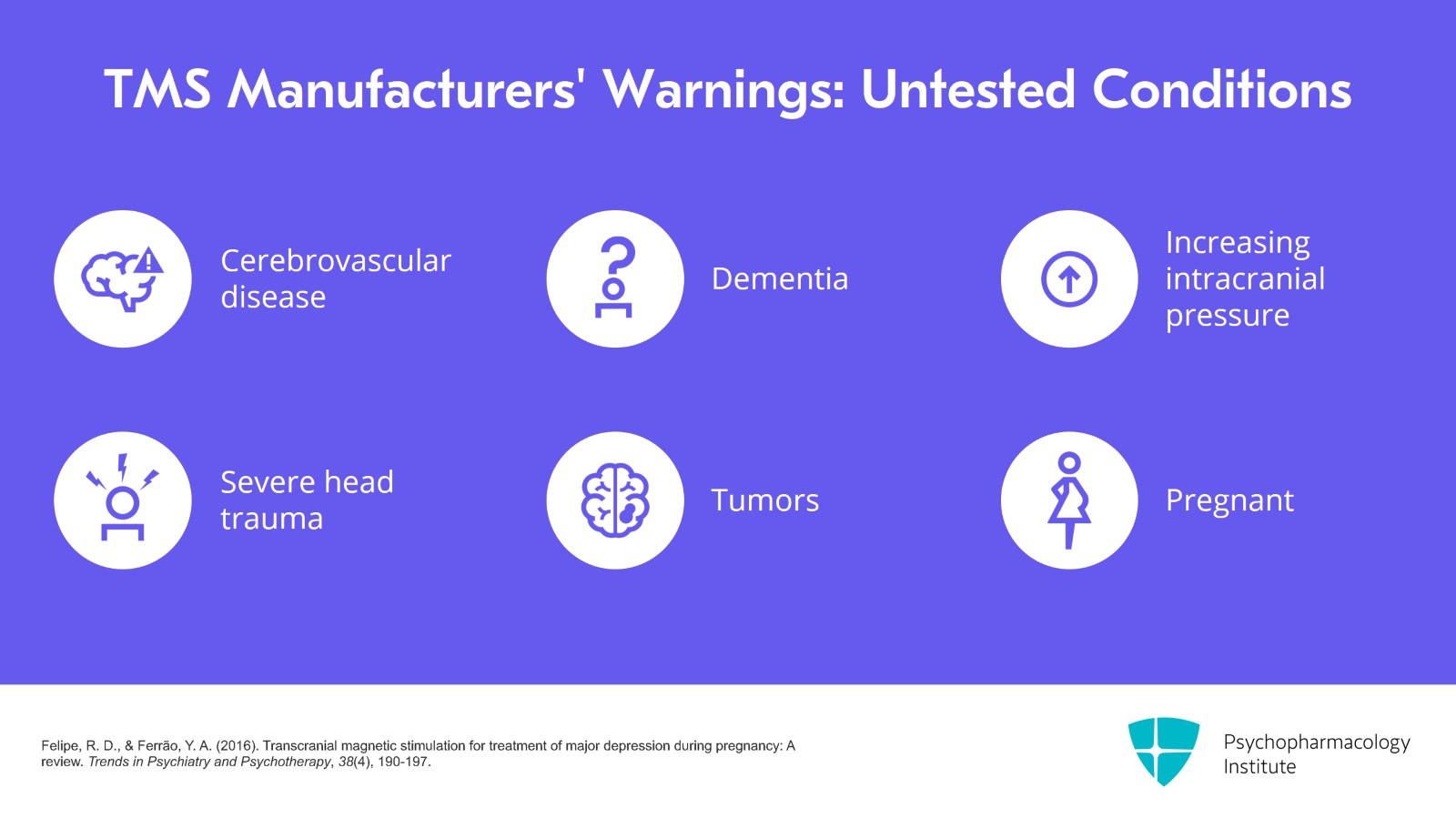
TMS manufacturers usually have several other cautions that TMS hasn't been tested in patients who have cerebrovascular disease, dementia, increasing intracranial pressure, severe head trauma or tumors. TMS has also not been formally tested in patients who were pregnant although there are a few case reports and case series of its safety.
References:
- Felipe, R. D., & Ferrão, Y. A. (2016). Transcranial magnetic stimulation for treatment of major depression during pregnancy: A review. Trends in Psychiatry and Psychotherapy, 38(4), 190-197.
Slide 10 of 24
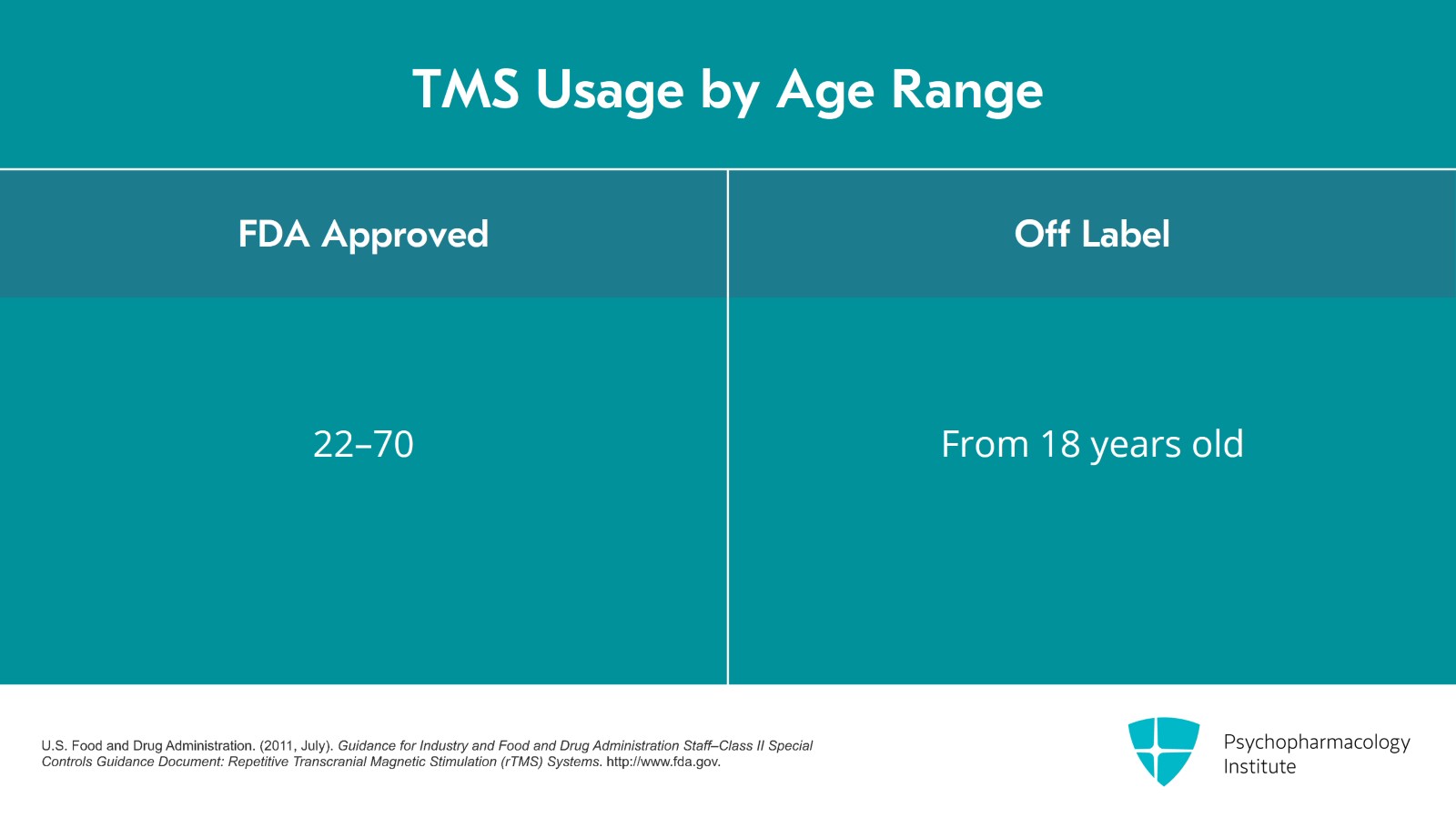
There's also off-label use of TMS. From the FDA perspective, the ages and diagnosis of patients for TMS should be what was tested in the clinical trials which got TMS approved. The ages of patients in the original clinical trial were 22 to 70. So officially, that's the on-label age range. However, insurance companies will usually cover ages 18 and older although I have seen some insurance companies that do have an upper age limit.
References:
- U.S. Food and Drug Administration. (2011, July). Guidance for Industry and Food and Drug Administration Staff–Class II Special Controls Guidance Document: Repetitive Transcranial Magnetic Stimulation (rTMS) Systems. http://www.fda.gov.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 11 of 24
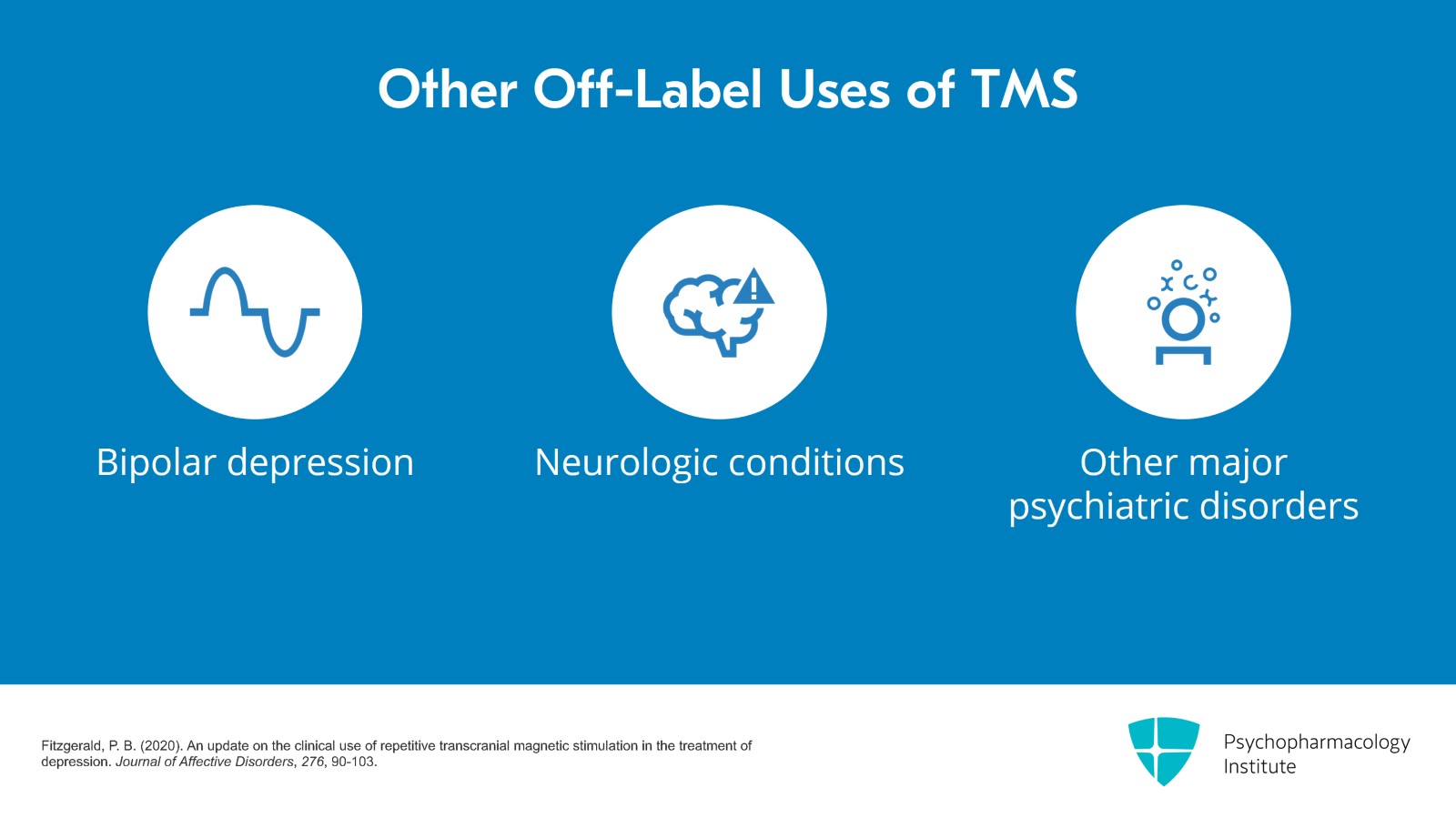
For the diagnosis, the original clinical trial only included patients with unipolar major depression so that became the FDA approval. TMS for bipolar depression is off-label. If there are any neurologic conditions, those were not tested in the clinical trials, so those are off-label as well or other major psychiatric disorders such as schizoaffective disorder. Just because it's off-label doesn't mean that it can't be safe.
References:
- Fitzgerald, P. B. (2020). An update on the clinical use of repetitive transcranial magnetic stimulation in the treatment of depression. Journal of Affective Disorders, 276, 90-103.
Slide 12 of 24
So for example, there are several studies showing TMS can be very helpful for bipolar depression and that it is safe and there's no major switch into mania. However, since it is off-label, insurance companies don't cover it.
References:
- Tavares, D. F., Myczkowski, M. L., Alberto, R. L., Valiengo, L., Rios, R. M., Gordon, P., De Sampaio-Junior, B., Klein, I., Mansur, C. G., Marcolin, M. A., Lafer, B., Moreno, R. A., Gattaz, W., Daskalakis, Z. J., & Brunoni, A. R. (2017). Treatment of bipolar depression with deep TMS: Results from a double-blind, randomized, parallel group, sham-controlled clinical trial. Neuropsychopharmacology, 42(13), 2593-2601.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 13 of 24
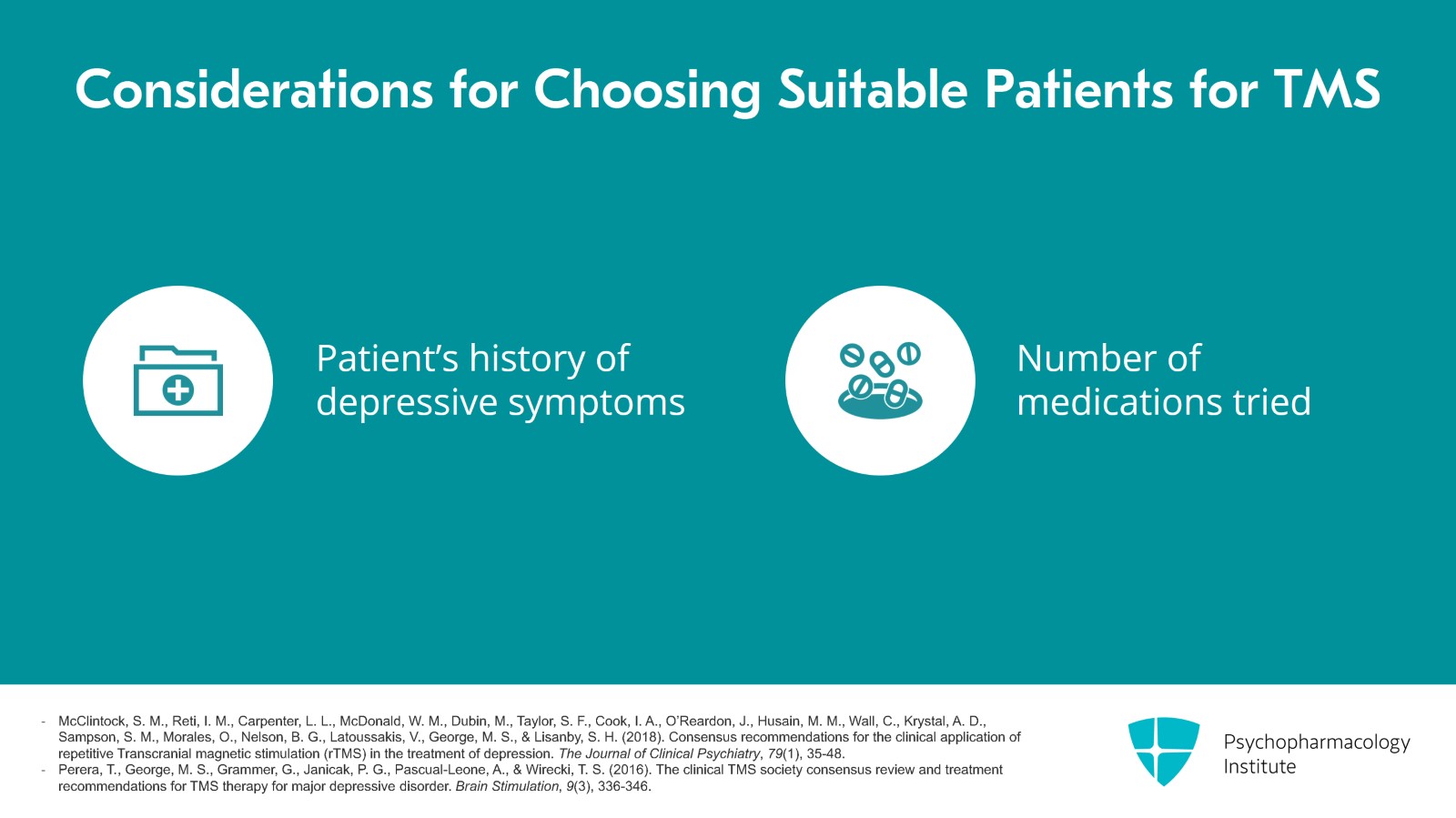
So considerations for how to choose a patient that would be good for TMS. It's important to take a good history of the patient's depression and symptoms. So some insurance medical necessity criteria will refer to the number of medications tried in the current episode of depression or they'll say the lifetime episodes.
References:
- McClintock, S. M., Reti, I. M., Carpenter, L. L., McDonald, W. M., Dubin, M., Taylor, S. F., Cook, I. A., O’Reardon, J., Husain, M. M., Wall, C., Krystal, A. D., Sampson, S. M., Morales, O., Nelson, B. G., Latoussakis, V., George, M. S., & Lisanby, S. H. (2018). Consensus recommendations for the clinical application of repetitive Transcranial magnetic stimulation (rTMS) in the treatment of depression. The Journal of Clinical Psychiatry, 79(1), 35-48.
- Perera, T., George, M. S., Grammer, G., Janicak, P. G., Pascual-Leone, A., & Wirecki, T. S. (2016). The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimulation, 9(3), 336-346.
Slide 14 of 24
Whenever I ask the patient, how long is your episode of depression, they'll usually tell me for a long time. My practical question to the patient is, when was the last time you didn't feel burdened by depression and sometimes they'll give me a clear answer and other times, the answer is still since I was age 11. But that's okay. It's whatever the patient feels. What's the severity and urgency of depression? That's another consideration.
References:
- McClintock, S. M., Reti, I. M., Carpenter, L. L., McDonald, W. M., Dubin, M., Taylor, S. F., Cook, I. A., O’Reardon, J., Husain, M. M., Wall, C., Krystal, A. D., Sampson, S. M., Morales, O., Nelson, B. G., Latoussakis, V., George, M. S., & Lisanby, S. H. (2018). Consensus recommendations for the clinical application of repetitive Transcranial magnetic stimulation (rTMS) in the treatment of depression. The Journal of Clinical Psychiatry, 79(1), 35-48.
- Perera, T., George, M. S., Grammer, G., Janicak, P. G., Pascual-Leone, A., & Wirecki, T. S. (2016). The clinical TMS society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimulation, 9(3), 336-346.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 15 of 24
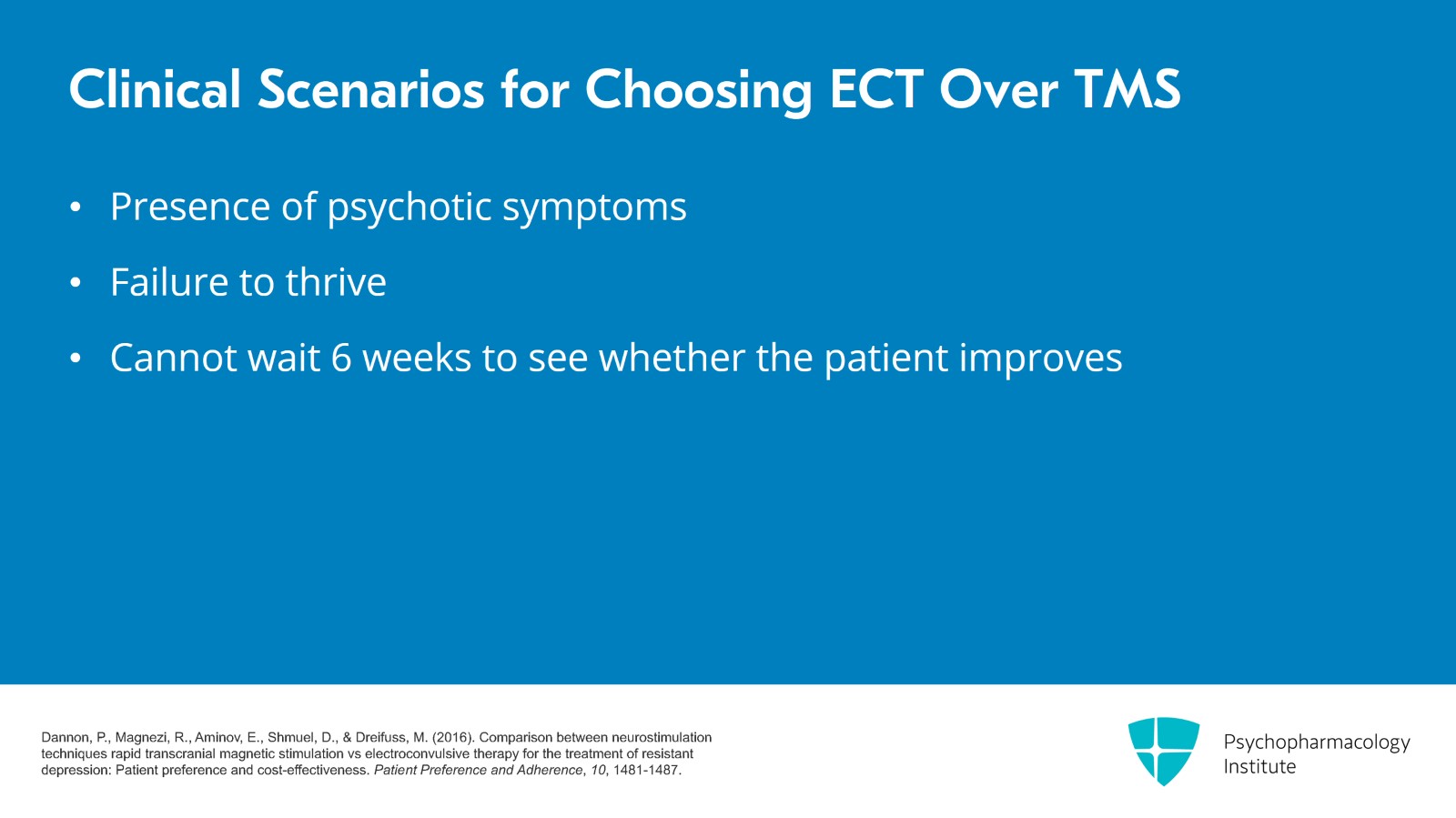
If there are any psychotic symptoms or failure to thrive such as not eating or drinking, I would recommend ECT instead of TMS. These are situations that you don't want to wait six weeks to see if the patient gets better. So ECT will usually get the patient better in two or three weeks.
References:
- Dannon, P., Magnezi, R., Aminov, E., Shmuel, D., & Dreifuss, M. (2016). Comparison between neurostimulation techniques rapid transcranial magnetic stimulation vs electroconvulsive therapy for the treatment of resistant depression: Patient preference and cost-effectiveness. Patient Preference and Adherence, 10, 1481-1487.
Slide 16 of 24
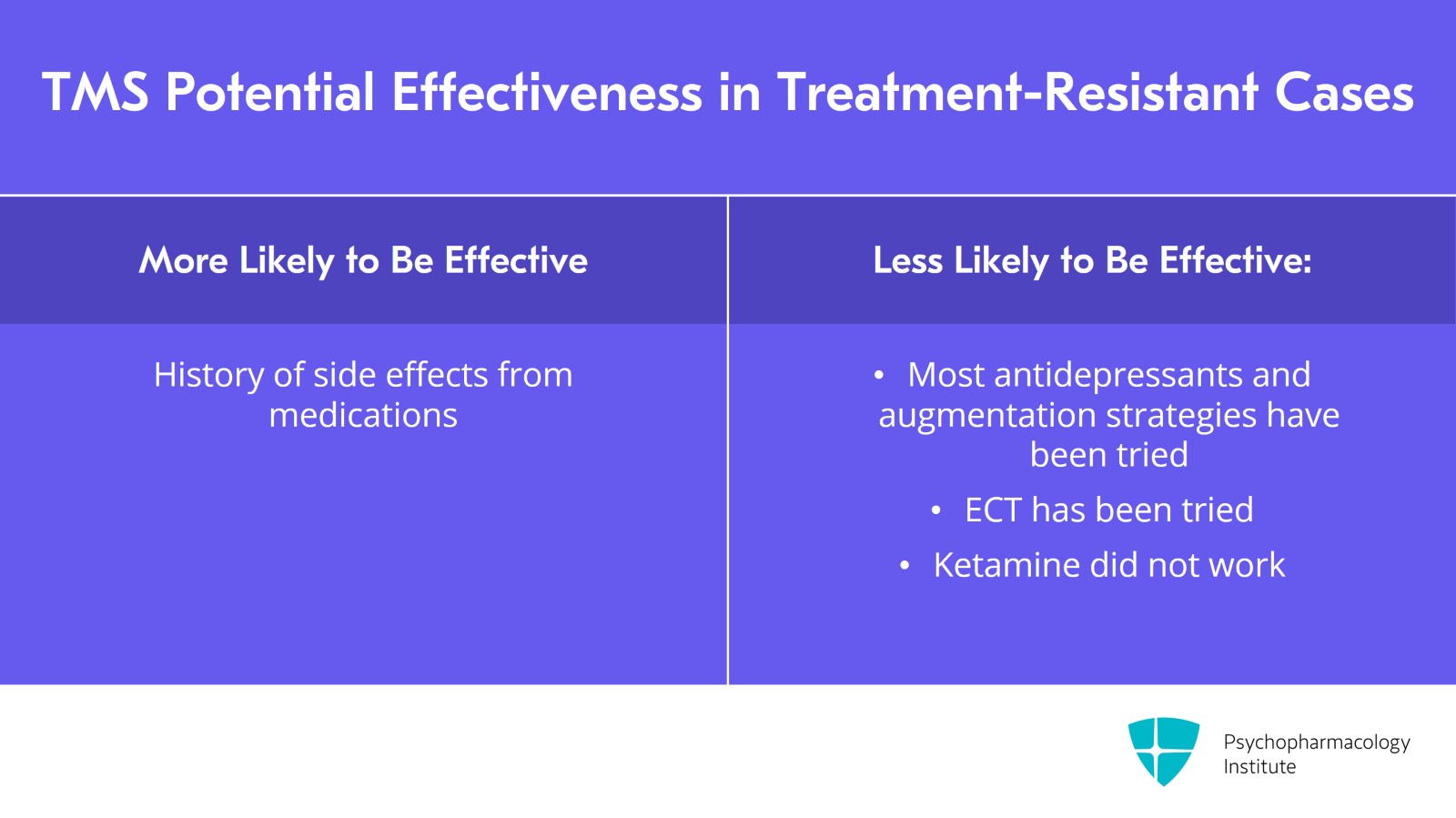
What's the treatment resistance? If a patient has been on most, all of our antidepressants and augmentation strategies, I'm less hopeful that TMS will be the answer to their depression. If there has been a lot of side effects with medications, TMS might be helpful and could offer patient hope that a nonmedication treatment can be helpful. If a patient has had ECT before, I'm also less hopeful that TMS will be helpful unless ECT was helpful and they didn't want to have ECT again because of side effects. If a patient had ketamine in the current episode which didn't help, I'm also less hopeful that TMS will be helpful.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 17 of 24
There are patients who don't respond to our biological treatments so I want to make sure that they're trying psychotherapy techniques such as acceptance and commitment.
Slide 18 of 24
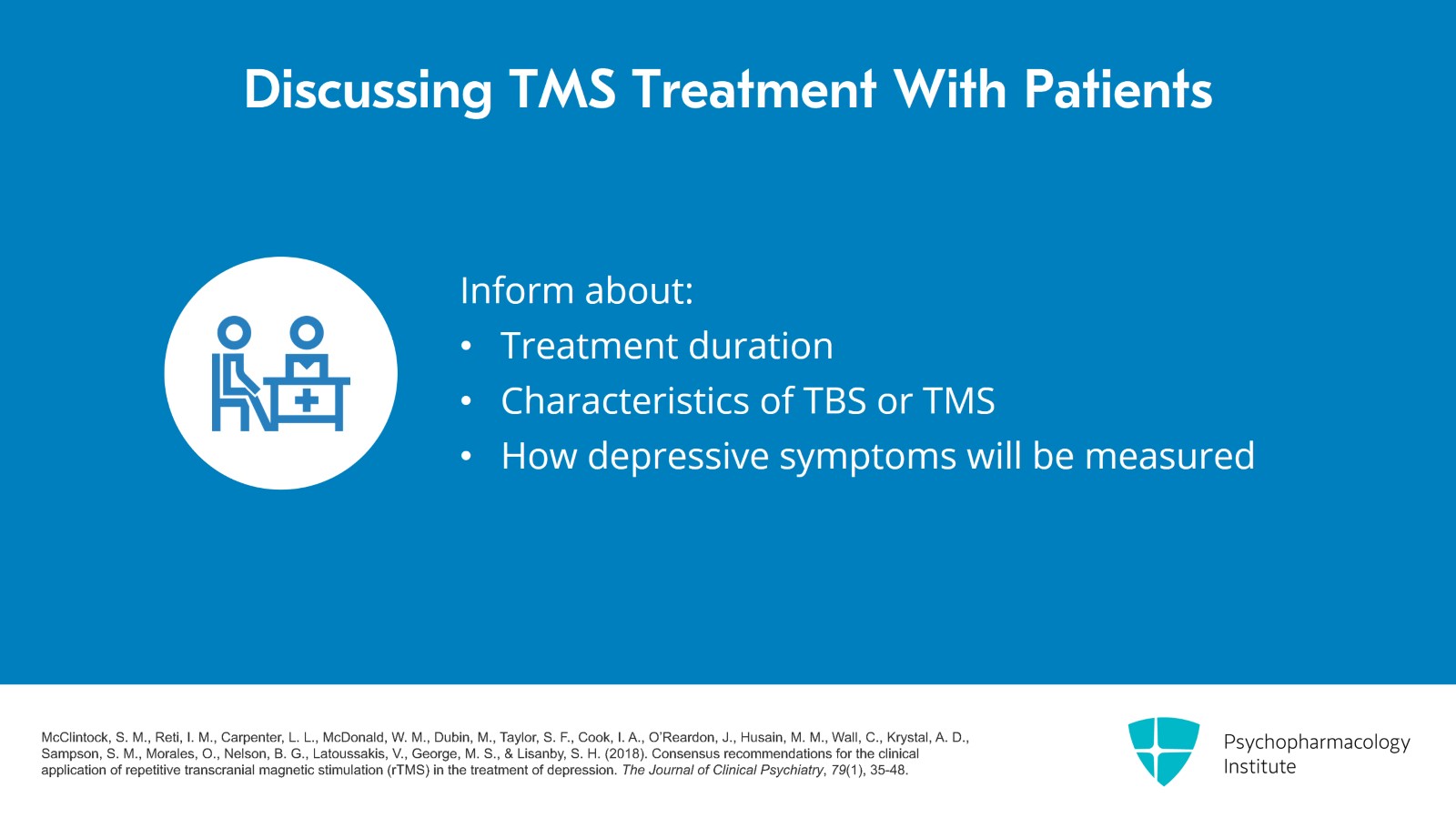
When I meet with patients, there are things that I discuss with them about TMS and I think these are important things to let people know about. So I review the treatment. I talk about the duration of treatment, that it's Monday through Friday for six weeks and we taper if there's a response. I discuss the use of TBS or TMS, that I usually use left unilateral treatments. I also discuss how are depressive symptoms going to be measured and what rating skills can be used.
References:
- McClintock, S. M., Reti, I. M., Carpenter, L. L., McDonald, W. M., Dubin, M., Taylor, S. F., Cook, I. A., O’Reardon, J., Husain, M. M., Wall, C., Krystal, A. D., Sampson, S. M., Morales, O., Nelson, B. G., Latoussakis, V., George, M. S., & Lisanby, S. H. (2018). Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. The Journal of Clinical Psychiatry, 79(1), 35-48.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 19 of 24
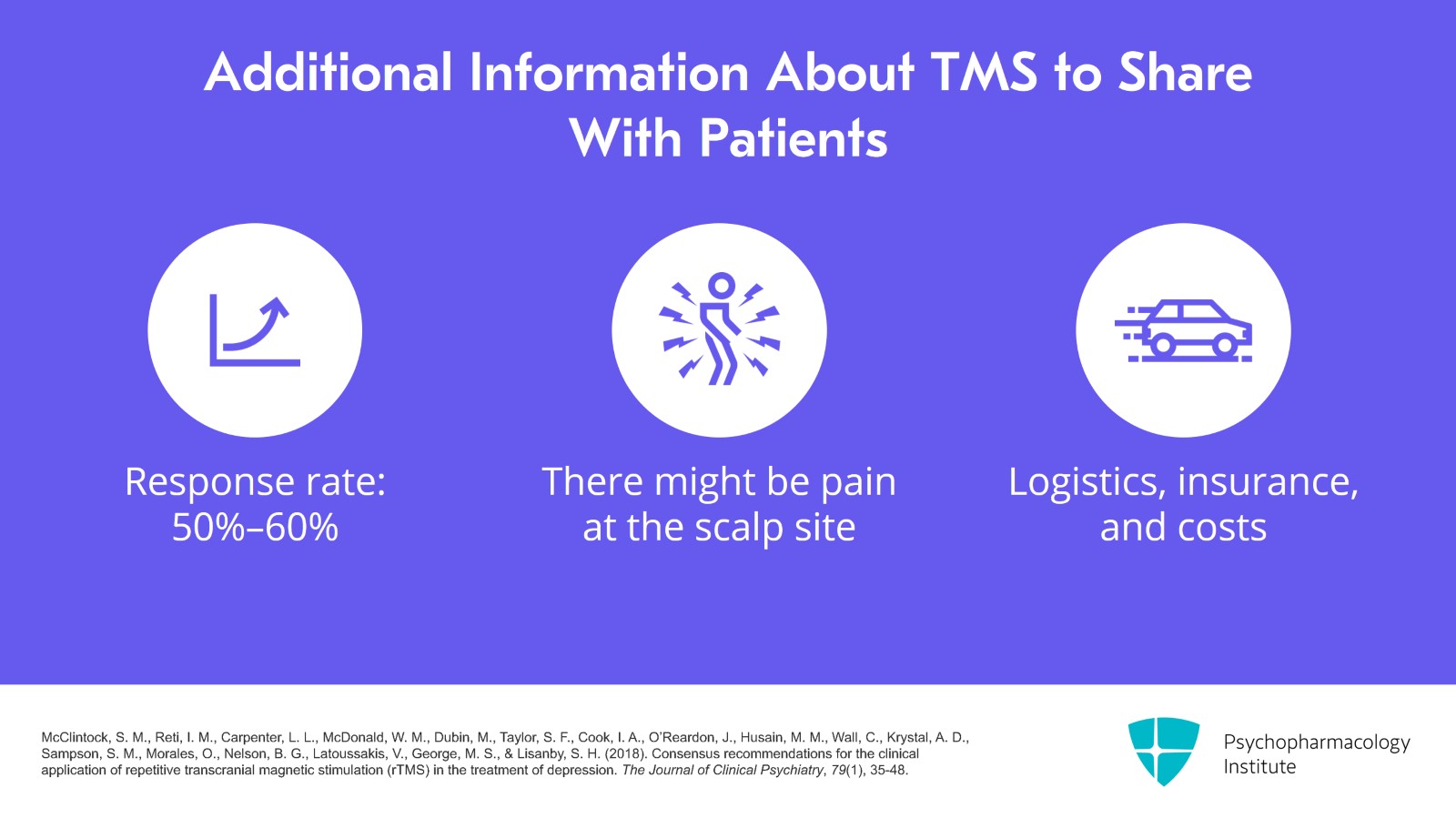
I review the potential benefits telling them that the response rate is 50% to 60% and also that it's going to hurt, that there is going to be pain with TMS at the scalp site. I review logistics such as you're going to have to drive here every day and also the insurance and the cost. The nice thing about TMS is patients can drive to the treatments themselves.
References:
- McClintock, S. M., Reti, I. M., Carpenter, L. L., McDonald, W. M., Dubin, M., Taylor, S. F., Cook, I. A., O’Reardon, J., Husain, M. M., Wall, C., Krystal, A. D., Sampson, S. M., Morales, O., Nelson, B. G., Latoussakis, V., George, M. S., & Lisanby, S. H. (2018). Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. The Journal of Clinical Psychiatry, 79(1), 35-48.
Slide 20 of 24
Then I'll review what happens after TMS, that about six months afterwards the relapse rate is about 50%, they should continue with medications and psychotherapy and that insurance doesn't cover maintenance TMS but it covers re-treatment if the mood holds up for at least three months.
References:
- Janicak, P. G., Nahas, Z., Lisanby, S. H., Solvason, H. B., Sampson, S. M., McDonald, W. M., Marangell, L. B., Rosenquist, P., McCall, W. V., Kimball, J., O’Reardon, J. P., Loo, C., Husain, M. H., Krystal, A., Gilmer, W., Dowd, S. M., Demitrack, M. A., & Schatzberg, A. F. (2010). Durability of clinical benefit with transcranial magnetic stimulation (TMS) in the treatment of pharmacoresistant major depression: Assessment of relapse during a 6-month, multisite, open-label study. Brain Stimulation, 3(4), 187-199.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 21 of 24
At some point, patients will ask about other nonmedication options for their treatment-resistant depression. I'll discuss the pros and cons of ECT, ketamine, and TMS with patients if they ask. Many of the factors have to do with cost, availability in the area, time involvement and whether a patient has someone to give them a ride to and from treatments. So these are all very practical considerations.
References:
- McIntyre, R. S., Rosenblat, J. D., Nemeroff, C. B., Sanacora, G., Murrough, J. W., Berk, M., Brietzke, E., Dodd, S., Gorwood, P., Ho, R., Iosifescu, D. V., Lopez Jaramillo, C., Kasper, S., Kratiuk, K., Lee, J. G., Lee, Y., Lui, L. M., Mansur, R. B., Papakostas, G. I., … Stahl, S. (2021). Synthesizing the evidence for ketamine and esketamine in treatment-resistant depression: An international expert opinion on the available evidence and implementation. American Journal of Psychiatry, 178(5), 383-399.
- Dannon, P., Magnezi, R., Aminov, E., Shmuel, D., & Dreifuss, M. (2016). Comparison between neurostimulation techniques rapid transcranial magnetic stimulation vs electroconvulsive therapy for the treatment of resistant depression: Patient preference and cost-effectiveness. Patient Preference and Adherence, 10, 1481-1487.
Slide 22 of 24
Key points. Get a good history of the patient's depression, the current episode, and the current and past treatments. Insurance medical necessity criteria frequently guides whether a patient's eligible for TMS.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 23 of 24
Contraindications to TMS include seizure history or metallic objects in the head. Practical aspects of TMS include logistics and insurance coverage.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.