Slides and Transcript
Slide 1 of 25
In this section of the talk, I’d like to talk a little bit about how medications pass into the breastmilk and how we understand that because in order to determine whether there could be any adverse effects from that, we need to understand that passage.
Slide 2 of 25
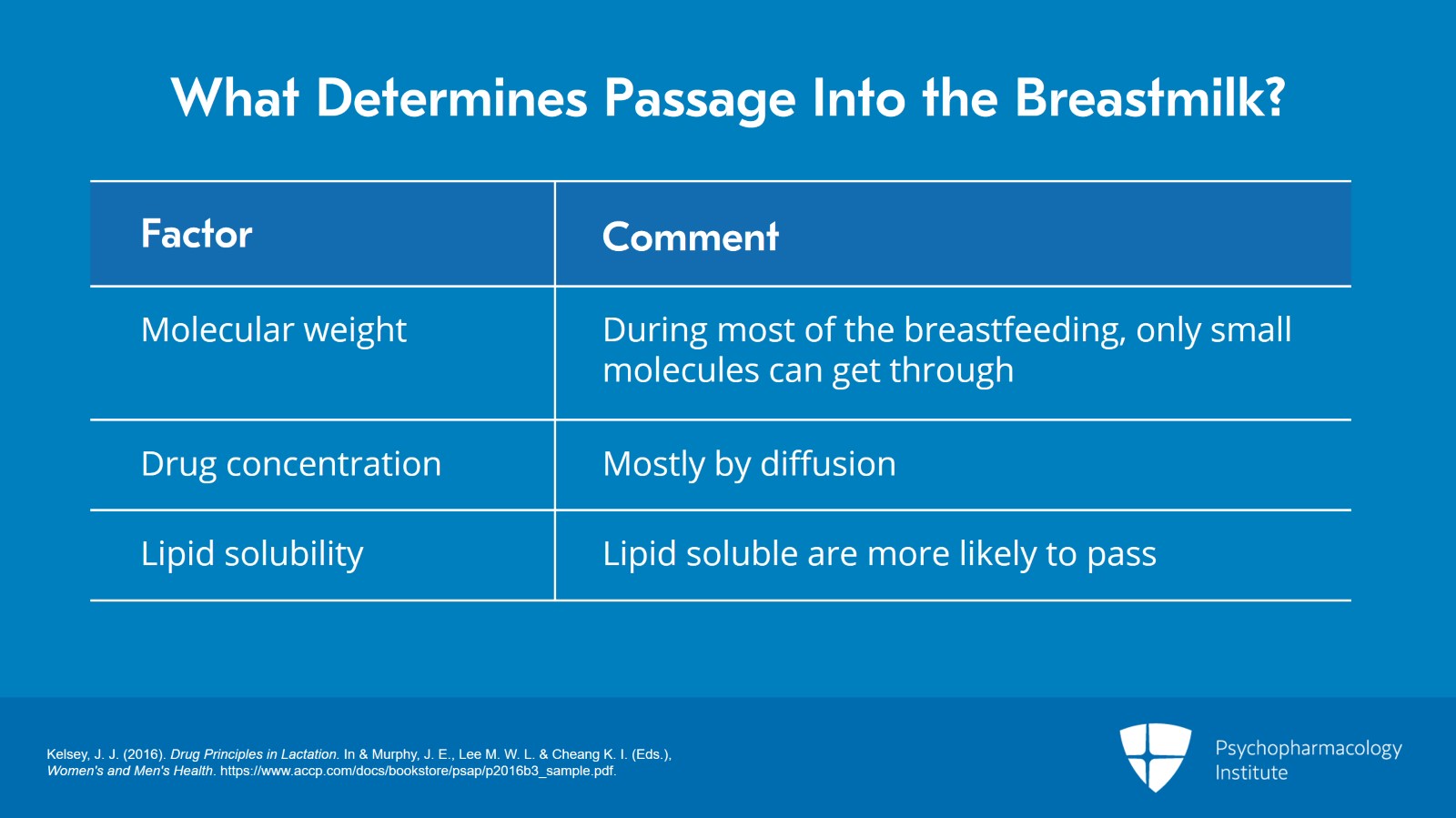
So what determines the passage into the breastmilk? Well, a number of things do. The first one I already mentioned in the previous video and that’s molecular weight. At the very beginning and very end of breastfeeding, large molecules can pass in easily but during most of breastfeeding only small molecules can get through. Those of up to 200 Da will pass easily. Sometimes, those up to 1000 Da are possible. Anything larger, much more difficult.
Drug concentration also affects what passes into the breastmilk. Mostly, drugs will pass in by diffusion, but some drugs actually require active transport.
Lipid solubility is another factor to consider. Remember that it’s a lipid membrane. So, anything lipid soluble is going to be much more likely to pass into the breastmilk.
References:
- Kelsey, J. J. (2016). Drug Principles in Lactation. In & Murphy, J. E., Lee M. W. L. & Cheang K. I. (Eds.), Women's and Men's Health. https://www.accp.com/docs/bookstore/psap/p2016b3_sample.pdf.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 3 of 25
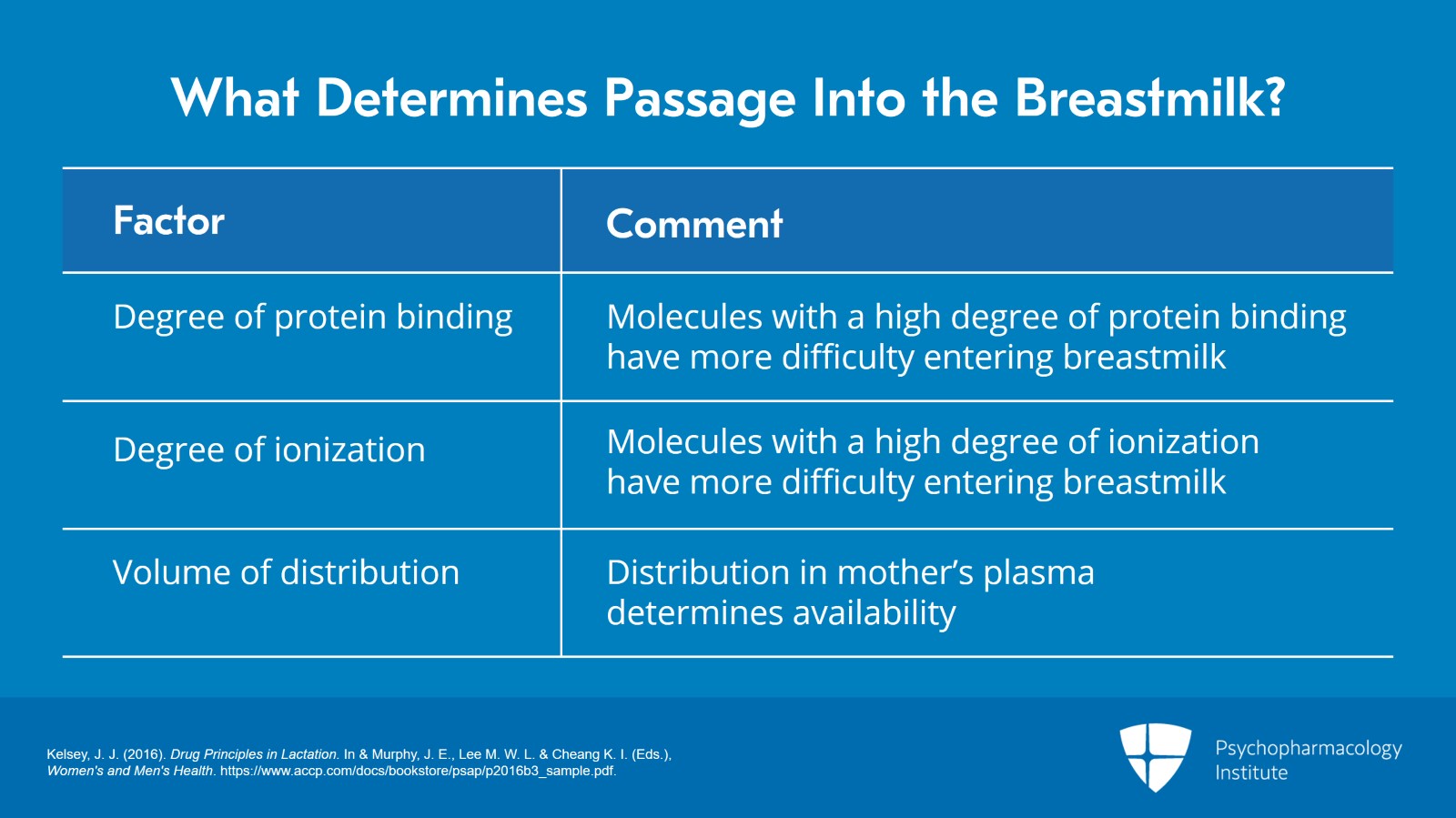
The degree of protein binding is also important. Molecules that have high protein binding will enter the breastmilk more difficult.
Similarly, the degree of ionization is important. Anything with a high degree of ionization enter the breastmilk more difficult.
The volume of distribution is important. So how that drug is distributed in the mother’s plasma will affect how much there is available to get into the breastmilk.
References:
- Kelsey, J. J. (2016). Drug Principles in Lactation. In & Murphy, J. E., Lee M. W. L. & Cheang K. I. (Eds.), Women's and Men's Health. https://www.accp.com/docs/bookstore/psap/p2016b3_sample.pdf.
Slide 4 of 25
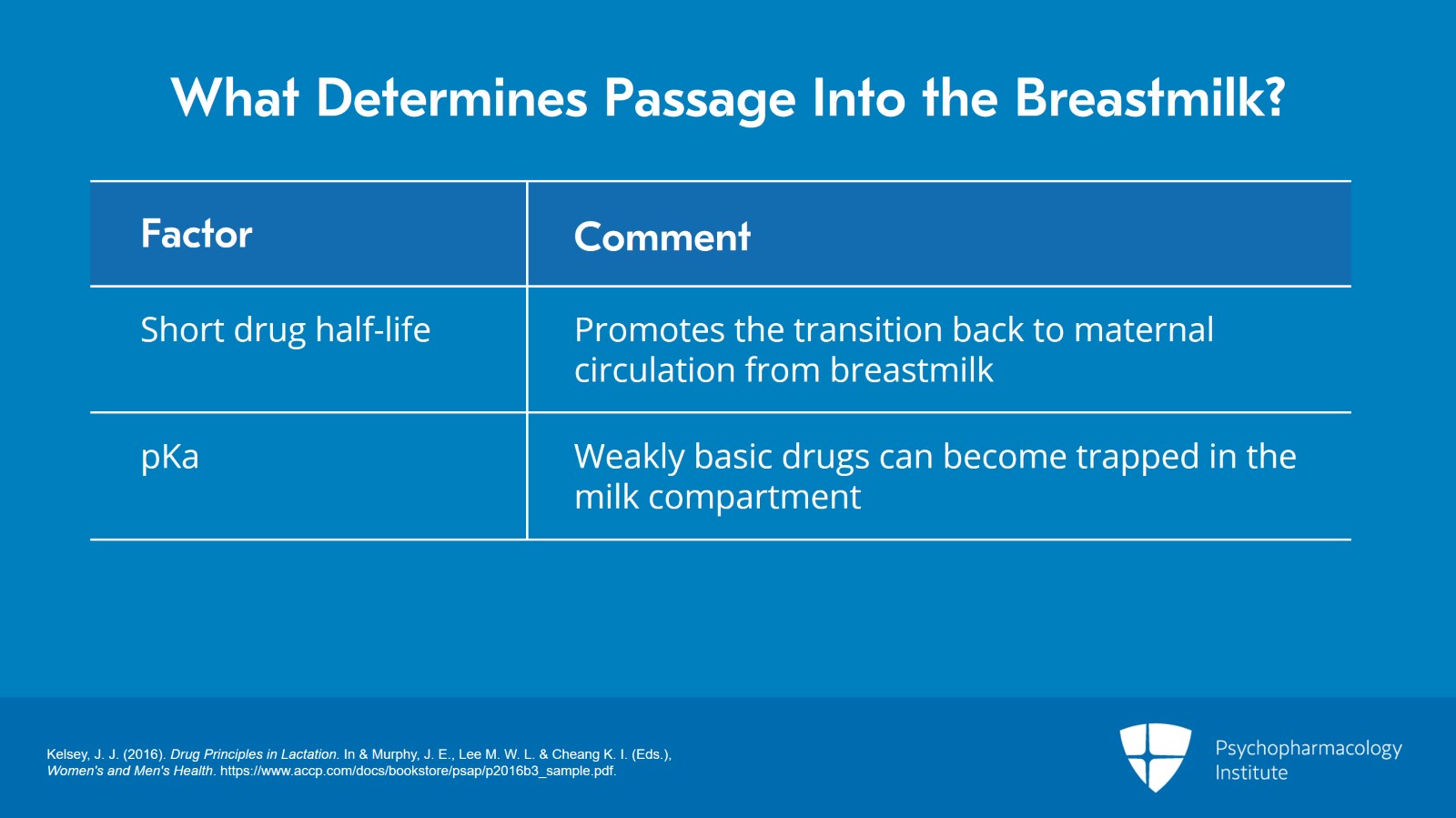
And the half-life of the drug. If the half-life is short, will actually promote passage back into the maternal circulation.
And finally, the pKa. Weakly basic drugs can become trapped in the milk compartment because it’s more acidic than the blood is. So, all of those things factor in. And so, any individual drug can have different combinations of all these factors that affect whether or not it will pass into the breastmilk.
References:
- Kelsey, J. J. (2016). Drug Principles in Lactation. In & Murphy, J. E., Lee M. W. L. & Cheang K. I. (Eds.), Women's and Men's Health. https://www.accp.com/docs/bookstore/psap/p2016b3_sample.pdf.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 5 of 25
It’s important also to remember some basic facts about excretion into the breastmilk. You know, I’ve had patients on a medication during pregnancy and then suddenly say, “Oh, but I don’t want to expose the baby through breastmilk,” and that actually doesn’t make any sense.
During pregnancy, most drugs will cross the placenta and the level of the drug in the fetal serum will equilibrate with that in the maternal serum. That means that the maternal dose and the maternal clearance are governing the infant’s serum level. They’re the only things governing the infant serum level.
References:
- Verstegen, R. H., Anderson, P. O., & Ito, S. (2020). Infant drug exposure via breast milk. British Journal of Clinical Pharmacology.
Slide 6 of 25
During lactation, by contrast, infants are exposed only to a fraction of the maternal dose. So, the infant dose via the milk and the infant clearance are therefore also involved in the governing the infant’s serum level.
References:
- Verstegen, R. H., Anderson, P. O., & Ito, S. (2020). Infant drug exposure via breast milk. British Journal of Clinical Pharmacology.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 7 of 25
So, during pregnancy, it’s only maternal factors. During lactation, it’s both infant and maternal factors so you’re actually having a much lower fraction of the drug during breastmilk.
What that means is if a woman was on a drug during pregnancy there’s no logical reason to stop that drug during breastfeeding.
References:
- Verstegen, R. H., Anderson, P. O., & Ito, S. (2020). Infant drug exposure via breast milk. British Journal of Clinical Pharmacology.
Slide 8 of 25
The reason for this is we have to remember the mammary gland is neither a major drug eliminating organ nor is it a reservoir. So, in other words, once the medication gets into the mammary gland, it’s not eliminated from the gland, but it also doesn’t hang on there.
The infant is exposed only to the amount of drug that gets excreted into the milk while the infant is feeding. So, it’s not the case that the drug is built up in the woman’s plasma goes into the milk and sits there waiting for the baby to feed. The baby is feeding on milk that’s produced while the baby is feeding. So, the amount that the baby is exposed to is the amount excreted while she is feeding.
References:
- Verstegen, R. H., Anderson, P. O., & Ito, S. (2020). Infant drug exposure via breast milk. British Journal of Clinical Pharmacology.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 9 of 25
It’s also really important to understand infant pharmacokinetics to have a sense of what these drugs are doing in the infant.
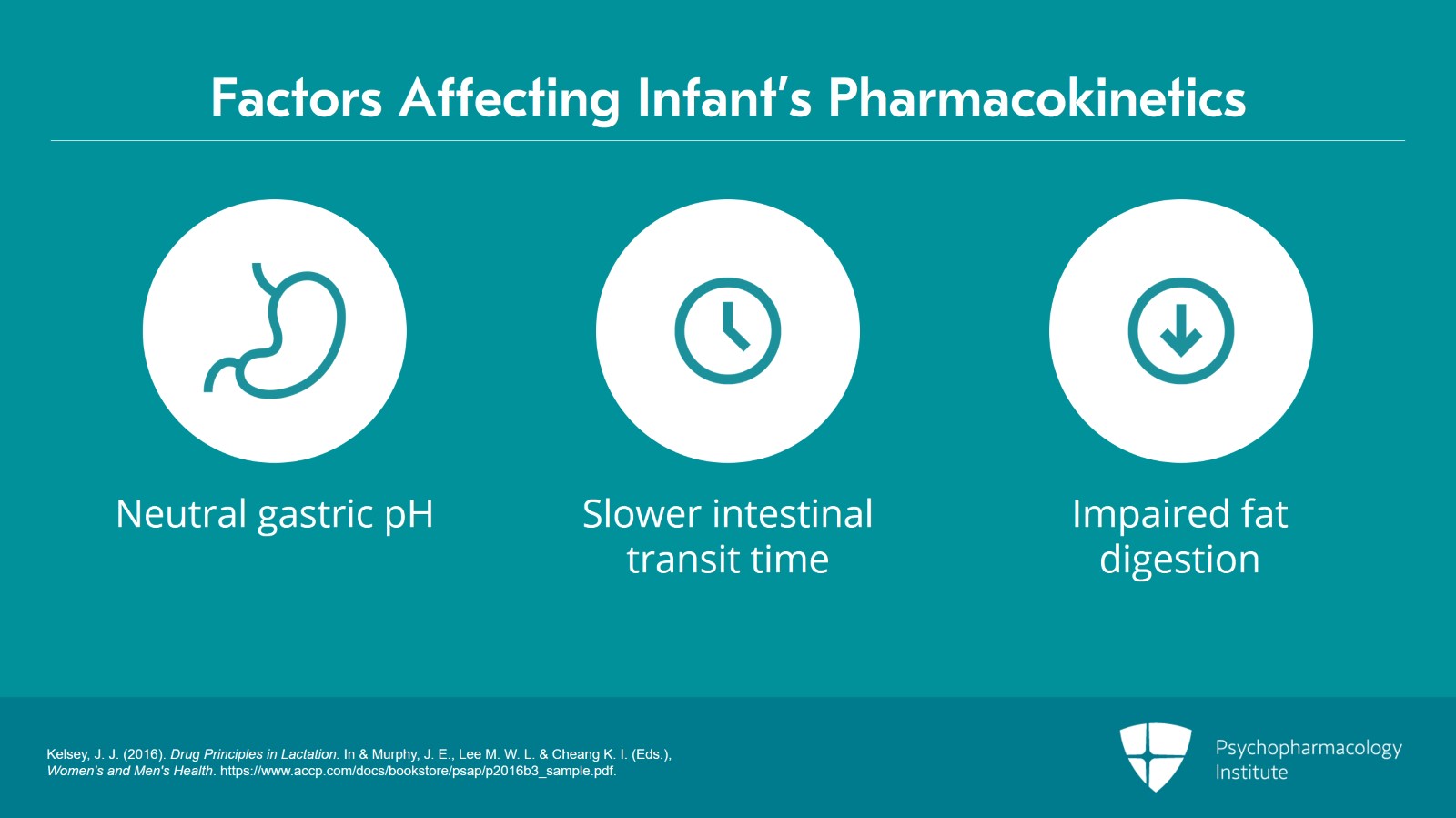
Remember that when the newborn is first born, gastric pH is neutral. It drops in that first day and then slowly rises back to neutral by about day 10. And that of course is going to affect the absorption of drugs.
Intestinal transit times are longer than in adults. There’s impaired fat digestion.
*References *
References:
- Kelsey, J. J. (2016). Drug Principles in Lactation. In & Murphy, J. E., Lee M. W. L. & Cheang K. I. (Eds.), Women's and Men's Health. https://www.accp.com/docs/bookstore/psap/p2016b3_sample.pdf.
Slide 10 of 25
The gut bacteria which are so important for maintaining the health of the baby actually are radically different depending on the mode of delivery. So, babies born via vaginal birth are going to have a different set of gut bacteria than those born via cesarean section.
Crucially, for the drugs that we use as psychiatrists, the blood-brain barrier is much more permeable in a newborn than in an adult. So that means that the drugs that we tend to use are actually some of the ones that most readily get to the infant’s brain.
*References *
References:
- Kelsey, J. J. (2016). Drug Principles in Lactation. In & Murphy, J. E., Lee M. W. L. & Cheang K. I. (Eds.), Women's and Men's Health. https://www.accp.com/docs/bookstore/psap/p2016b3_sample.pdf.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 11 of 25
Also, many CYP enzymes have lower activity in the infant than they do in adults including CYP450 1A2, 3A4, 2C19 and 2D6, all of which are crucial for metabolizing drugs that we use.
Finally, all of these things combined make up the fact that elimination rates are actually lower in infants.
So, what you have to remember is that although you have to consider the amount of the drug that’s getting to the infant, you also have to realize that clearance and metabolism of the drug are different in infants than in children.
References:
- Kelsey, J. J. (2016). Drug Principles in Lactation. In & Murphy, J. E., Lee M. W. L. & Cheang K. I. (Eds.), Women's and Men's Health. https://www.accp.com/docs/bookstore/psap/p2016b3_sample.pdf.
Slide 12 of 25
Another important consideration is to weigh the risks of using the drug compared to the risks of not using the drug. You know, a lot of people think of this as “let me weigh the risks against the benefits.” But that’s not actually the most accurate way to think when you’re talking about a postpartum woman and child because the weighing the risk of the drug to infant and mother must be weighed against the risk of the untreated illness to both infant and mother. The need for the drug by the mother is a crucial point here.
We have to remember the potential adverse effects on the mother and the family system of failing to treat the illness.
References:
- Sachs, H. C. (2013). The transfer of drugs and therapeutics into human breast milk: An update on selected topics. Pediatrics, 132(3), e796-e809.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 13 of 25
We have to think about the mother’s access to and her response to nonpharmacological treatment. Some women may not have access to psychotherapy, some women may not respond to it so a medication may be the best choice.
We also have to remember the effect of the drug on milk production, the amount of the drug excreted into human milk.
References:
- Sachs, H. C. (2013). The transfer of drugs and therapeutics into human breast milk: An update on selected topics. Pediatrics, 132(3), e796-e809.
Slide 14 of 25
The extent of the oral absorption by the infant, any potential adverse effects on the infant from the drug and the age of the infant including prematurity. That’s a crucial thing to consider.
So, all of these factors are what we have to weigh in when we’re thinking about the risks of using the drug compared to the risks of not using the drug.
References:
- Sachs, H. C. (2013). The transfer of drugs and therapeutics into human breast milk: An update on selected topics. Pediatrics, 132(3), e796-e809.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 15 of 25
Remember also that infant intake and infant clearance are both crucial parameters to assess the infant dose. In those first few days after birth, clearance is very low but remember so is intake. That newborn stomach was only 5 to 7 mL of intake at a time, right?
Premature babies may not have the same clearance abilities as full-term babies and that’s a crucial factor.
Babies with kidney or liver dysfunction may have trouble clearing certain medications that rely on those organs. And then as the infant matures and adds other sources of nutrients, intake of the drug will go down as the percentage of milk in the diet is replaced by other foods.
References:
- Sachs, H. C. (2013). The transfer of drugs and therapeutics into human breast milk: An update on selected topics. Pediatrics, 132(3), e796-e809.
Slide 16 of 25
All of this may seem like an overwhelming amount of information but fortunately there are some great resources out there to learn about breastfeeding safety.
It would never be possible for one physician to remember the details of all the research on breastfeeding with any particular drug. So, we look it up. I look them up all the time even though this is my specialty.
References:
- Drugs and lactation database: (LactMed). (2006). National Library of Medicine. https://www.ncbi.nlm.nih.gov/books/NBK501922/?report=reader
- Home Page. MotherToBaby. (2021, February 2). http://www.mothertobaby.org/.
- Welcome to Reprotox. Reprotox. (n.d.). https://reprotox.org/.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 17 of 25
It’s also important to remember though that what you can learn from the literature is information about how those drugs get into the breastmilk and case reports of any adverse effects.
We actually have no research that weighs the relative risk to the infant against the relative risk to the mother or baby of not using the drugs. That’s the key clinical point and the research cannot help you with that.
References:
- Drugs and lactation database: (LactMed). (2006). National Library of Medicine. https://www.ncbi.nlm.nih.gov/books/NBK501922/?report=reader
- Home Page. MotherToBaby. (2021, February 2). http://www.mothertobaby.org/.
- Welcome to Reprotox. Reprotox. (n.d.). https://reprotox.org/.
Slide 18 of 25
In fact, groups that weigh in on the safety of drugs in breastfeeding often have only the infant in mind. For example, the American Academy of Pediatrics has a statement weighing on the safety of certain drugs in breastfeeding but they’re weighing that purely from what the risk may be to the baby without considering the risk to the mother or to the family system of not treating that psychiatric illness.
References:
- Sachs, H. C. (2013). The transfer of drugs and therapeutics into human breast milk: An update on selected topics. Pediatrics, 132(3), e796-e809.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 19 of 25
Several resources offer information about drugs and passage into the breastmilk. Some of the ones that I recommend include MotherToBaby. That’s a website. It’s just mothertobaby.org which has patient friendly fact sheets in both English and Spanish on the use of a number of different drugs in both pregnancy and lactation.
Reprotox which is a subscription-only service that you have to pay to subscribe to, but it has very detailed information on the use of all drugs in lactation and in pregnancy as well.
And then there’s a book, Hale’s Medications and Mother’s Milk which has really good resource on explaining how medications get into the milk and talking through what the adverse risks might be for different medications.
References:
- Home Page. MotherToBaby. (2021, February 2). http://www.mothertobaby.org/.
- Welcome to Reprotox. Reprotox. (n.d.). https://reprotox.org/.
- Hale, T. W. (2020). Hale's Medications & Mothers' Milk: A manual of Lactational pharmacology. Springer Publishing Company.
Slide 20 of 25
The most comprehensive source and the one that I go to most often is called LactMed. It’s part of the National Library of Medicine and it includes a summary of use of nearly all drugs in lactation. It gives information on maternal and infant levels when they’re known. It reviews the effects on infants, and it reviews the effects of the drug on milk production and often gives information on alternative drugs. It’s available as a website. I’m told that the app that they used to have is not now being kept up to date, but the website is and that’s an important thing to remember.
References:
- Drugs and lactation database: (LactMed). (2006). National Library of Medicine. https://www.ncbi.nlm.nih.gov/books/NBK501922/?report=reader
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 21 of 25
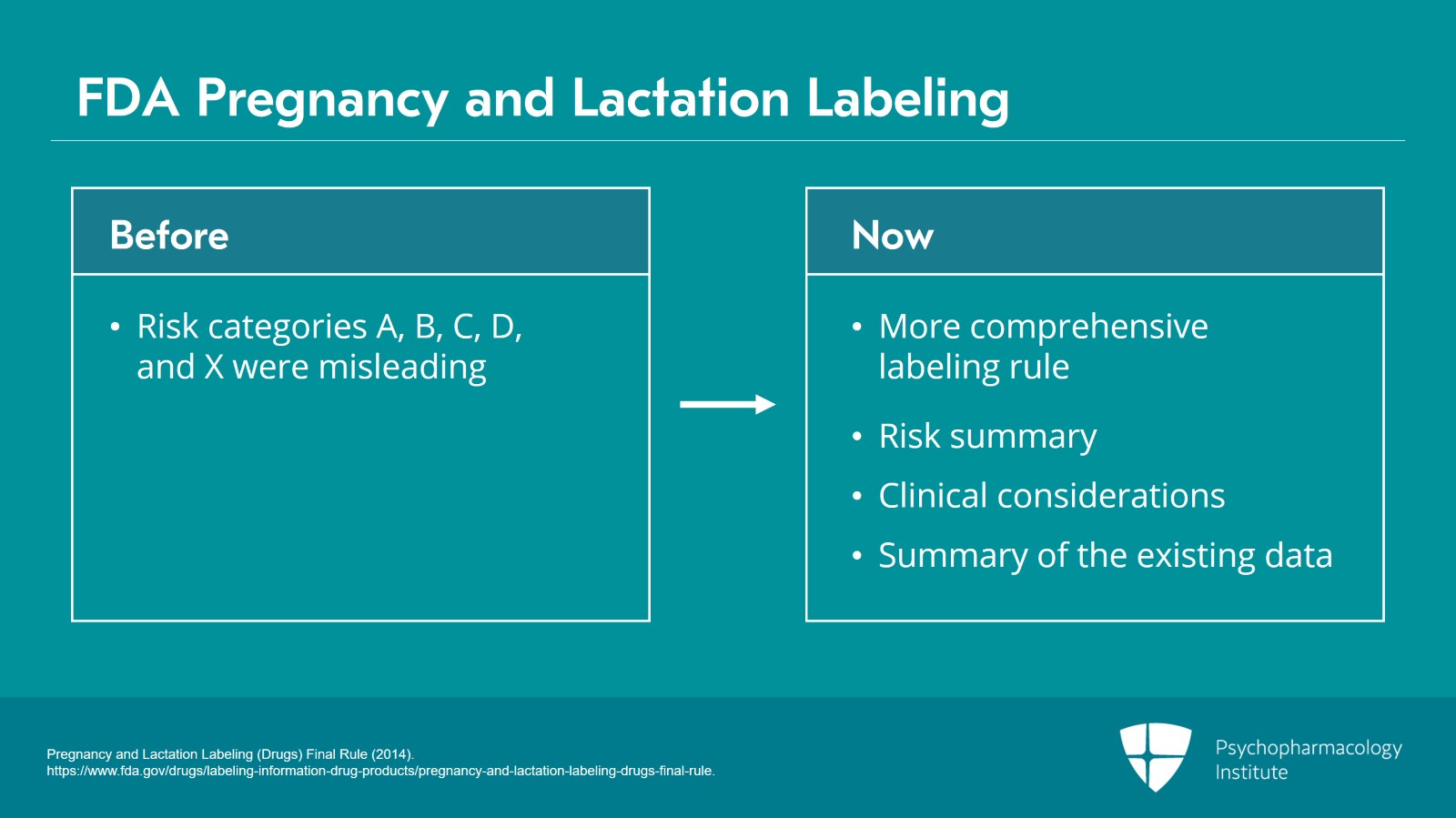
It’s also important to remember that the FDA has recently changed its pregnancy and lactation labeling.
So, we used to have a set of pregnancy risk categories, A, B, C, D, X, that were extremely misleading and led a lot of people to decline to use medications because they didn’t understand what that labeling meant.
There’s now a much more comprehensive pregnancy and lactation labeling rule that replaces those outmoded categories and it contains a risk summary that includes both the effects on the infants and effects on milk production. It includes clinical considerations such as information on how to minimize exposure and offers a summary of the existing data. It’s a huge improvement on the old categories.
References:
- Pregnancy and Lactation Labeling (Drugs) Final Rule (2014). https://www.fda.gov/drugs/labeling-information-drug-products/pregnancy-and-lactation-labeling-drugs-final-rule.
Slide 22 of 25
The problem is it leaves the decision with you, the clinician. People liked the old categories because they felt it was a grade. “Oh, this drug gets an A. I should use that drug.” But because people used it that way, it took out a lot of the nuance and it wasn’t in fact very accurate. So, this labeling rule is accurate, but it requires the clinician to really think about and consider the literature by summarizing it all.
References:
- Pregnancy and Lactation Labeling (Drugs) Final Rule (2014). https://www.fda.gov/drugs/labeling-information-drug-products/pregnancy-and-lactation-labeling-drugs-final-rule.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 23 of 25
So key points for this section of the talk. Remember that many factors about the drug including molecular weight, lipid solubility, and many others will determine how much of that drug gets passed into the breastmilk.
There are key factors of infant pharmacokinetics and metabolism such as intestinal transit time, elimination rates, and the activity of various CYP enzymes that will affect how that drug that’s ingested through the milk affects the infant.
Slide 24 of 25
Any decision made about treatment during breastfeeding must weigh the risks to the mother and infant of untreated illness against the risk to the infant of the drug. You know, a lot of the literature out there will tell you what the risk to the infant of the drug is but very little will weigh those risks together and that’s a crucial decision that you as a clinician have to make.
Finally, there are some great resources for information about drugs and lactation, my special favorite being LactMed from the National Library of Medicine. But again, remember these are focused on the risk to the infant and won’t consider the risks to the mother and family system of not using the drug.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.