Slides and Transcript
Slide 1 of 12
In the next section, we'll examine lamotrigine's effect on QTc prolongation and QRS widening as these were the two main concerns highlighted by the FDA warning.
Slide 2 of 12
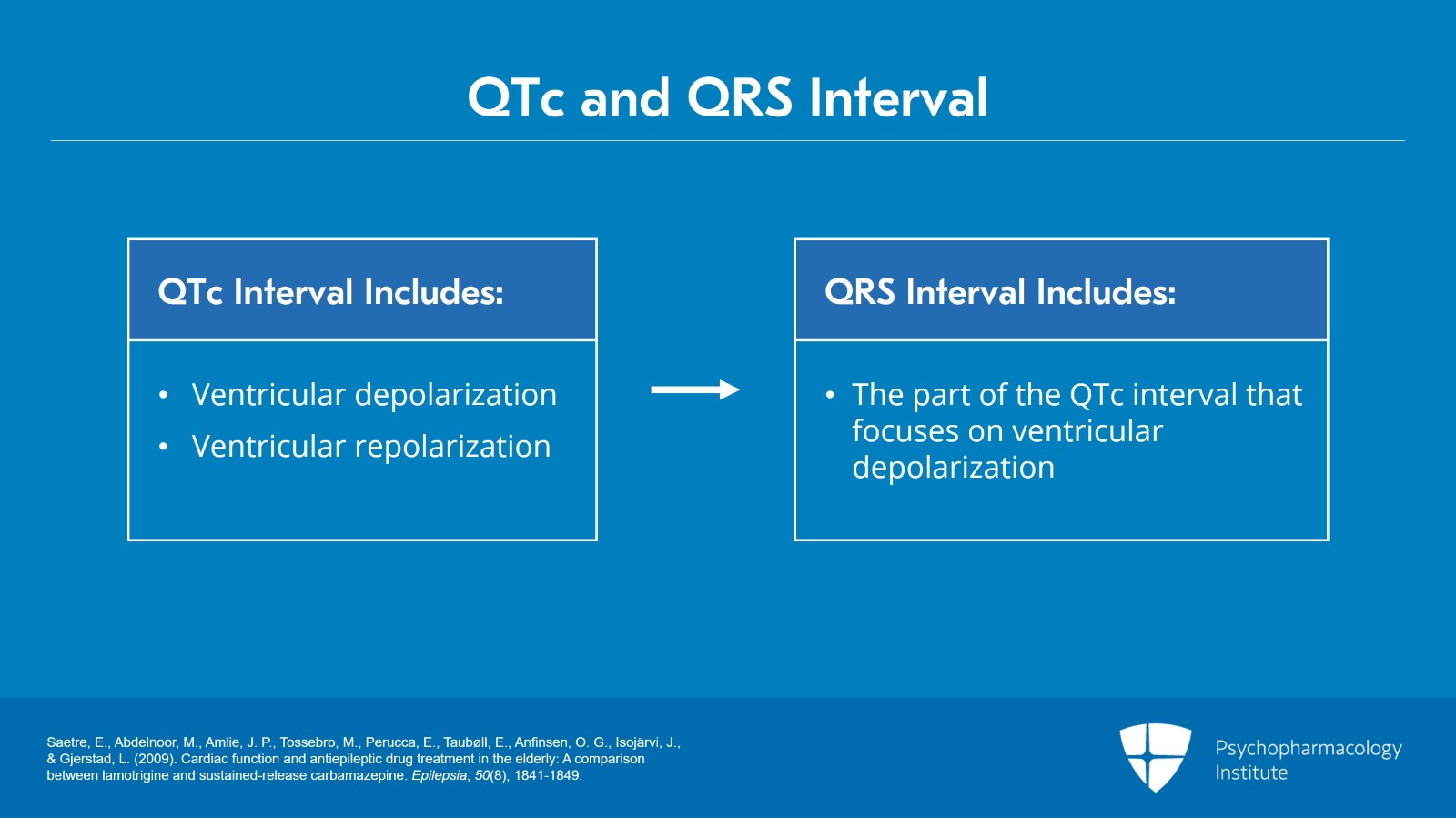
So as a reminder, when we think about the QTc interval, that includes ventricular depolarization and ventricular repolarization. When we think about the QRS interval, that's a part of the QTc interval but it's the part that just focuses on ventricular depolarization.
References:
- Saetre, E., Abdelnoor, M., Amlie, J. P., Tossebro, M., Perucca, E., Taubøll, E., Anfinsen, O. G., Isojärvi, J., & Gjerstad, L. (2009). Cardiac function and antiepileptic drug treatment in the elderly: A comparison between lamotrigine and sustained-release carbamazepine. Epilepsia, 50(8), 1841-1849.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 3 of 12
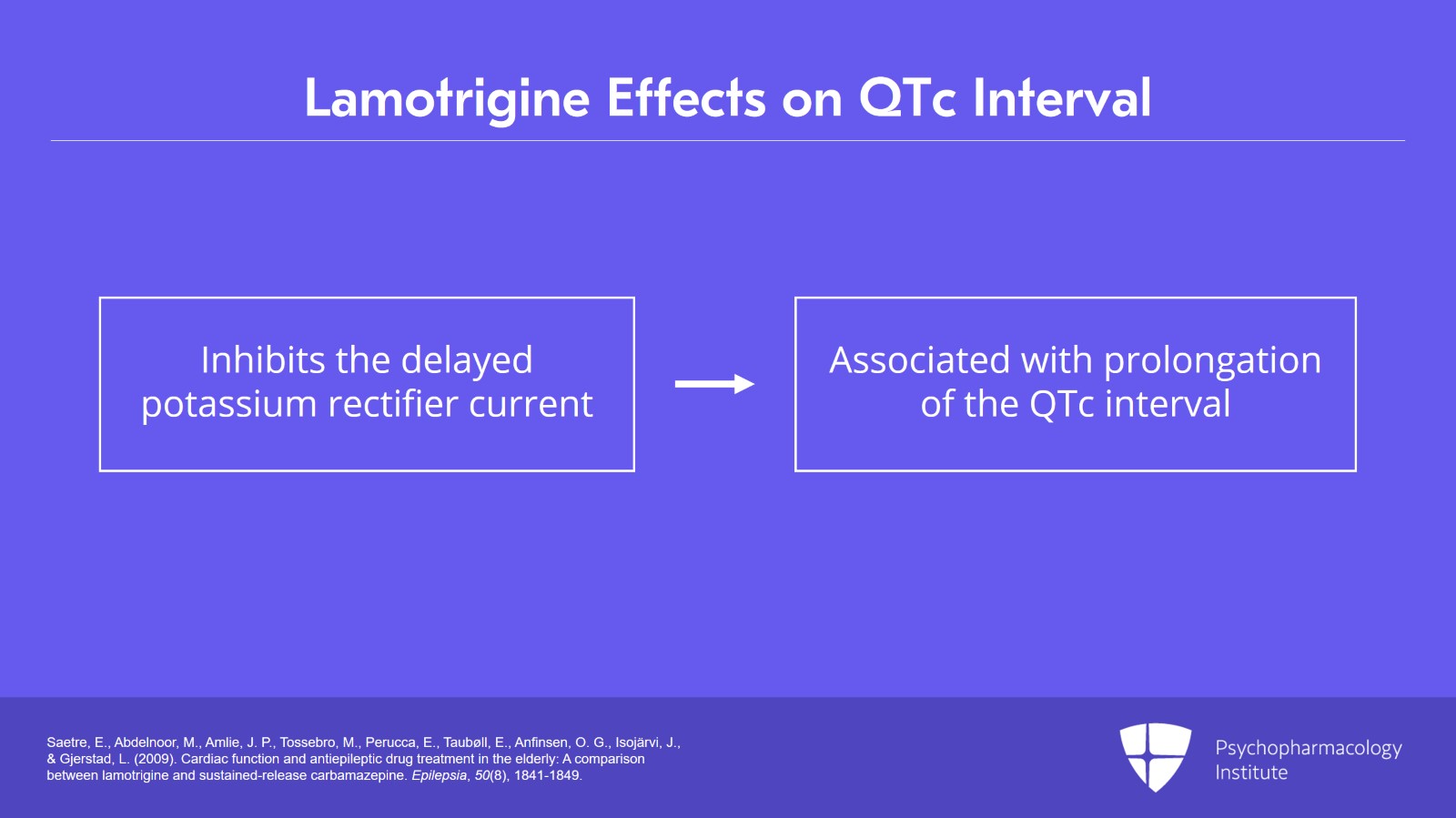
With regard to QTc prolongation, it's true that lamotrigine does inhibit the delayed potassium rectifier current in vitro and it's this channel which is most commonly associated with prolongation of the QTc interval in humans.
References:
- Saetre, E., Abdelnoor, M., Amlie, J. P., Tossebro, M., Perucca, E., Taubøll, E., Anfinsen, O. G., Isojärvi, J., & Gjerstad, L. (2009). Cardiac function and antiepileptic drug treatment in the elderly: A comparison between lamotrigine and sustained-release carbamazepine. Epilepsia, 50(8), 1841-1849.
Slide 4 of 12
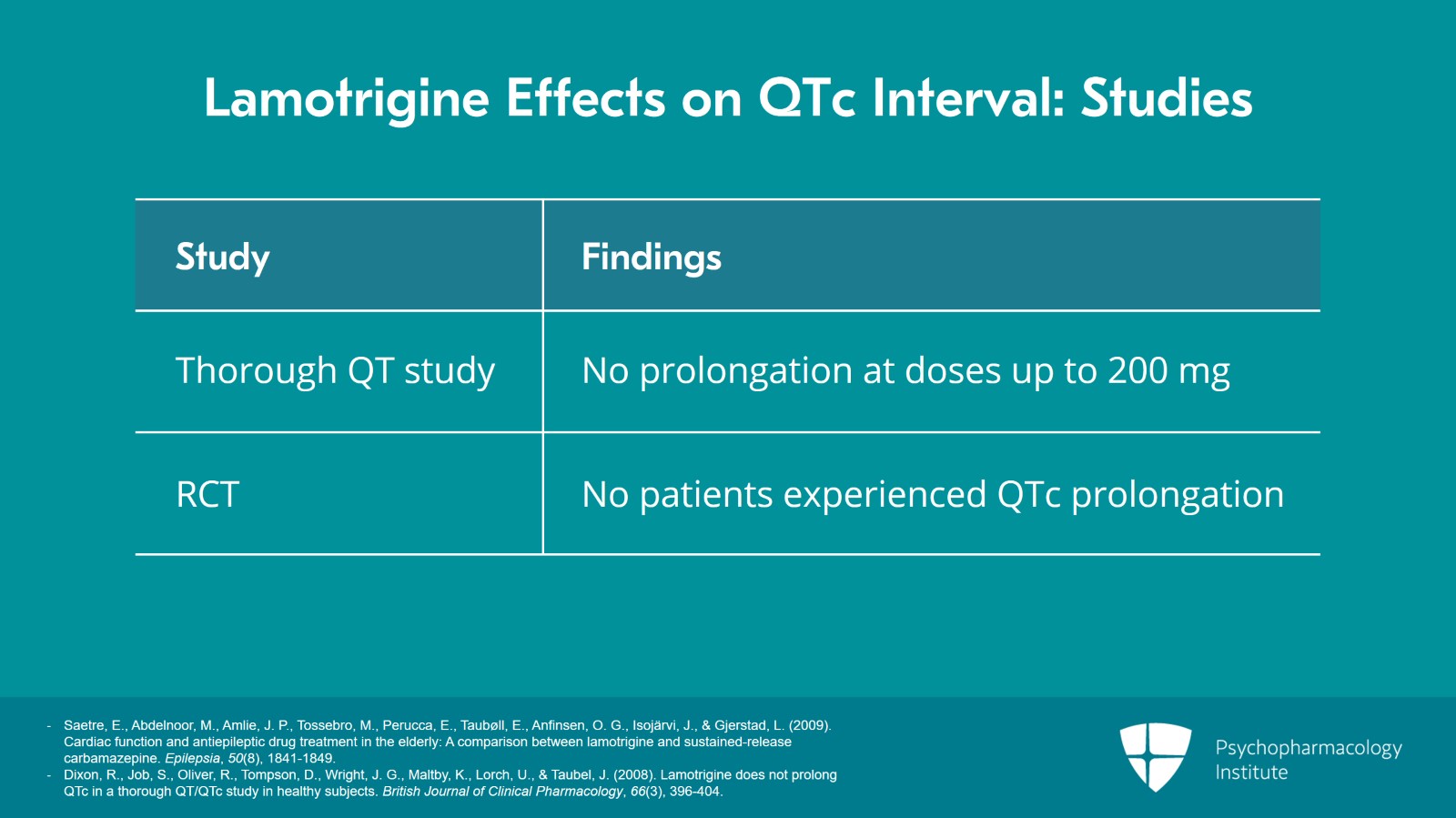
However, a thorough QT study with lamotrigine showed no prolongation at doses up to 200 mg. And as a reminder, that's the gold standard for investigating whether a medication prolongs the QTc. In a thorough QT study, the medication is compared to both placebo and an agent that is known to prolong the QTc and lamotrigine showed no prolongation in that study. Furthermore, a randomized controlled trial found that no patients experienced QTc prolongation while on lamotrigine.
References:
- Saetre, E., Abdelnoor, M., Amlie, J. P., Tossebro, M., Perucca, E., Taubøll, E., Anfinsen, O. G., Isojärvi, J., & Gjerstad, L. (2009). Cardiac function and antiepileptic drug treatment in the elderly: A comparison between lamotrigine and sustained-release carbamazepine. Epilepsia, 50(8), 1841-1849.
- Dixon, R., Job, S., Oliver, R., Tompson, D., Wright, J. G., Maltby, K., Lorch, U., & Taubel, J. (2008). Lamotrigine does not prolong QTc in a thorough QT/QTc study in healthy subjects. British Journal of Clinical Pharmacology, 66(3), 396-404.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 5 of 12
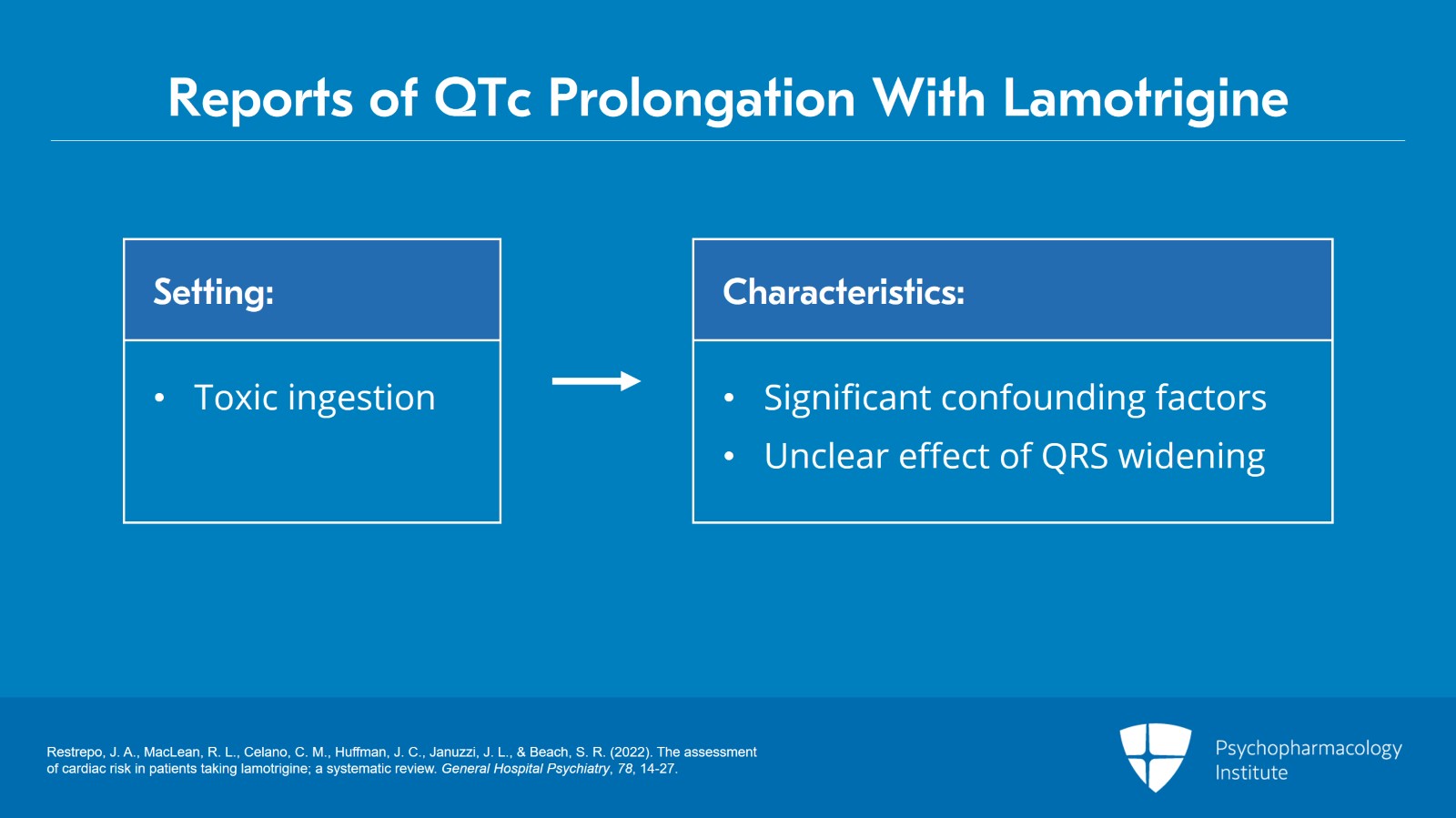
Now, there are some reports of QTc prolongation in the setting of toxic ingestion but all of those reports have significant confounding factors and an unclear effect of QRS widening which we'll get to in a minute.
References:
- Restrepo, J. A., MacLean, R. L., Celano, C. M., Huffman, J. C., Januzzi, J. L., & Beach, S. R. (2022). The assessment of cardiac risk in patients taking lamotrigine; a systematic review. General Hospital Psychiatry, 78, 14-27.
Slide 6 of 12
Overall, there's really no good evidence to suggest that lamotrigine causes significant QTc prolongation at therapeutic doses and I would not be concerned about that as a real risk in patients prescribed lamotrigine.
References:
- Restrepo, J. A., MacLean, R. L., Celano, C. M., Huffman, J. C., Januzzi, J. L., & Beach, S. R. (2022). The assessment of cardiac risk in patients taking lamotrigine; a systematic review. General Hospital Psychiatry, 78, 14-27.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 7 of 12
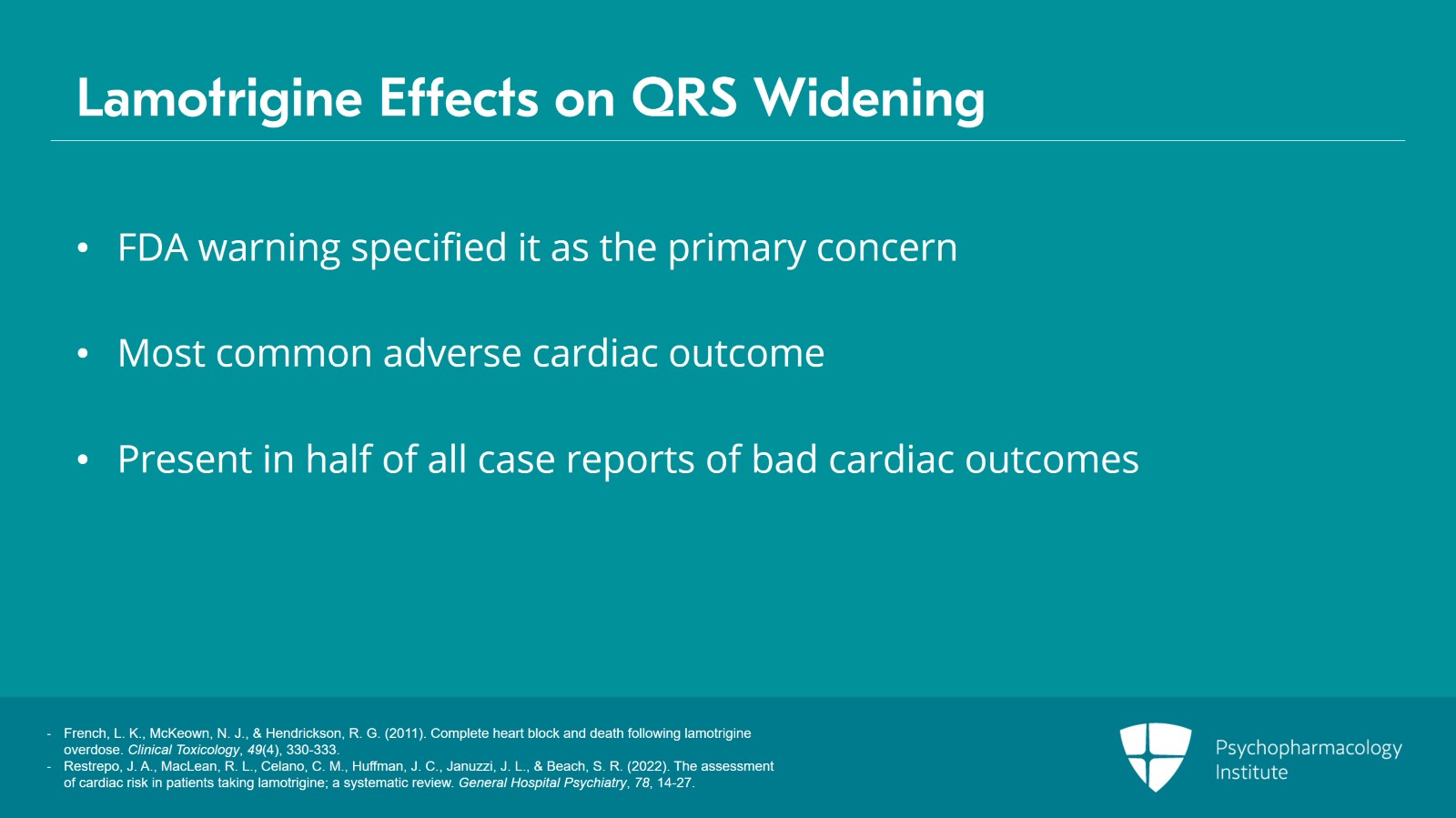
Let's shift now to think about QRS widening. The FDA warning specified QRS widening as the primary concern related to lamotrigine and the medication has been known for decades to act as a sodium channel blocker and we know that sodium channel blockers do cause QRS widening. QRS widening is in fact the most common adverse cardiac outcome documented in the case report literature with regard to lamotrigine and it's present in half of all case reports that documented some bad cardiac outcome.
References:
- French, L. K., McKeown, N. J., & Hendrickson, R. G. (2011). Complete heart block and death following lamotrigine overdose. Clinical Toxicology, 49(4), 330-333.
- Restrepo, J. A., MacLean, R. L., Celano, C. M., Huffman, J. C., Januzzi, J. L., & Beach, S. R. (2022). The assessment of cardiac risk in patients taking lamotrigine; a systematic review. General Hospital Psychiatry, 78, 14-27.
Slide 8 of 12
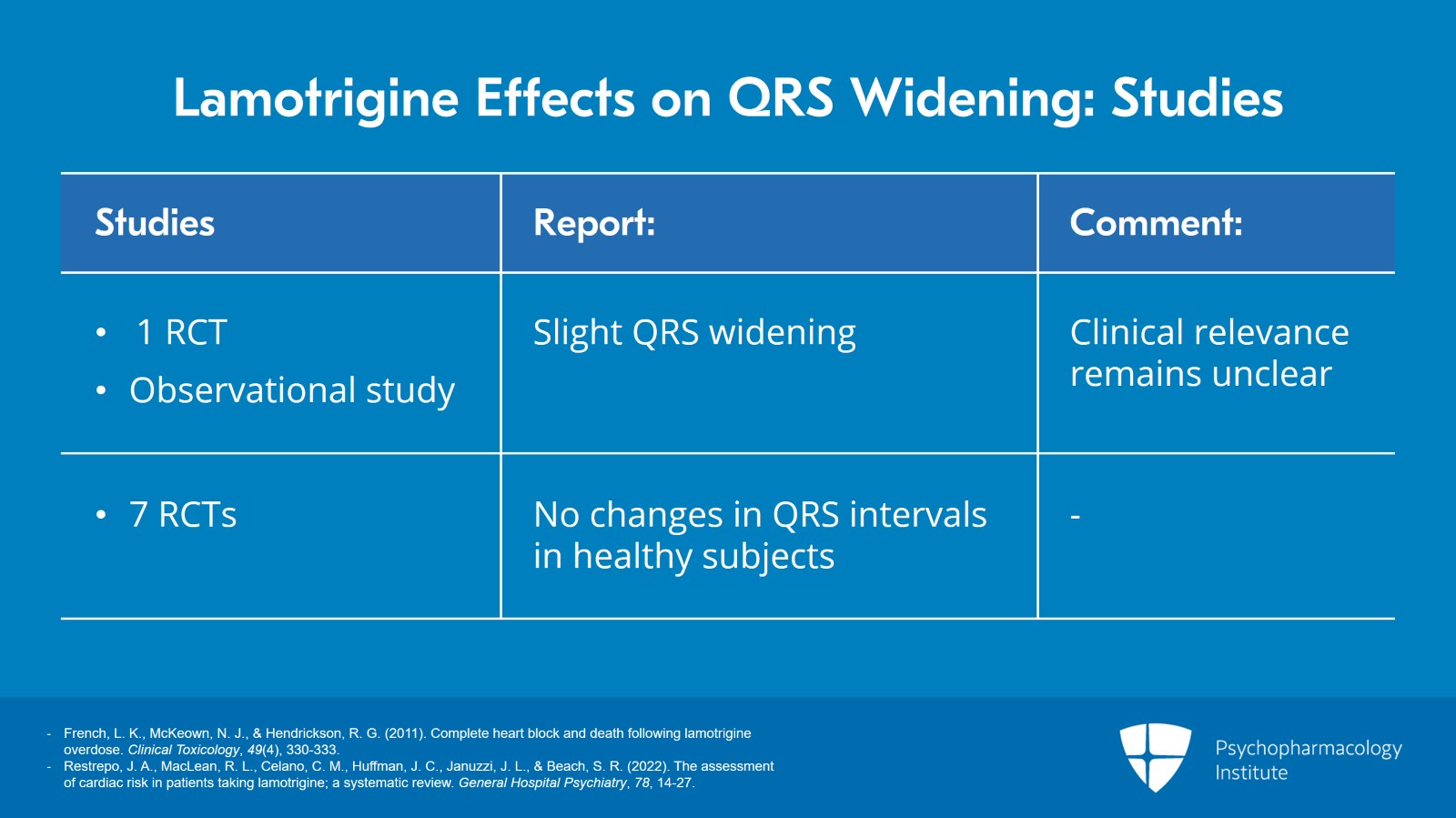
In a randomized trial involving older patients and also with two patients in an observational study of lamotrigine toxicity, slight QRS widening was reported. Now, the clinical relevance of this widening remains unclear so the randomized trial found an average prolongation of only 3.5 ms and many of the case reports noted that the QRS widening amounted to less than a total of 120 ms though there were some notable exceptions where the QRS interval got up to 214 ms. Furthermore, we have at least seven randomized trials that did not report any change in QRS intervals in healthy subjects even though that was measured as one of the parameters.
References:
- French, L. K., McKeown, N. J., & Hendrickson, R. G. (2011). Complete heart block and death following lamotrigine overdose. Clinical Toxicology, 49(4), 330-333.
- Restrepo, J. A., MacLean, R. L., Celano, C. M., Huffman, J. C., Januzzi, J. L., & Beach, S. R. (2022). The assessment of cardiac risk in patients taking lamotrigine; a systematic review. General Hospital Psychiatry, 78, 14-27.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 9 of 12
And overall, we think the risk of QRS widening with lamotrigine is similar to but less severe than with tricyclic antidepressants which are also known sodium channel blockers.
References:
- French, L. K., McKeown, N. J., & Hendrickson, R. G. (2011). Complete heart block and death following lamotrigine overdose. Clinical Toxicology, 49(4), 330-333.
- Restrepo, J. A., MacLean, R. L., Celano, C. M., Huffman, J. C., Januzzi, J. L., & Beach, S. R. (2022). The assessment of cardiac risk in patients taking lamotrigine; a systematic review. General Hospital Psychiatry, 78, 14-27.
Slide 10 of 12
So to summarize some key points from this section, there's really no evidence to suggest that lamotrigine causes QTc prolongation at therapeutic doses. Lamotrigine does appear to cause some QRS widening probably related to its sodium channel blocking effects but the overall risk is less significant than that with tricyclic antidepressants.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 11 of 12
And in general, the effect of QRS widening is unlikely to be clinically significant except perhaps in cases of significant overdose and toxicity.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.