Slides and Transcript
Slide 1 of 19
So in this section, we’ll continue to discuss second-generation antipsychotic LAIs in clinical practice focusing on paliperidone and risperidone.
Slide 2 of 19
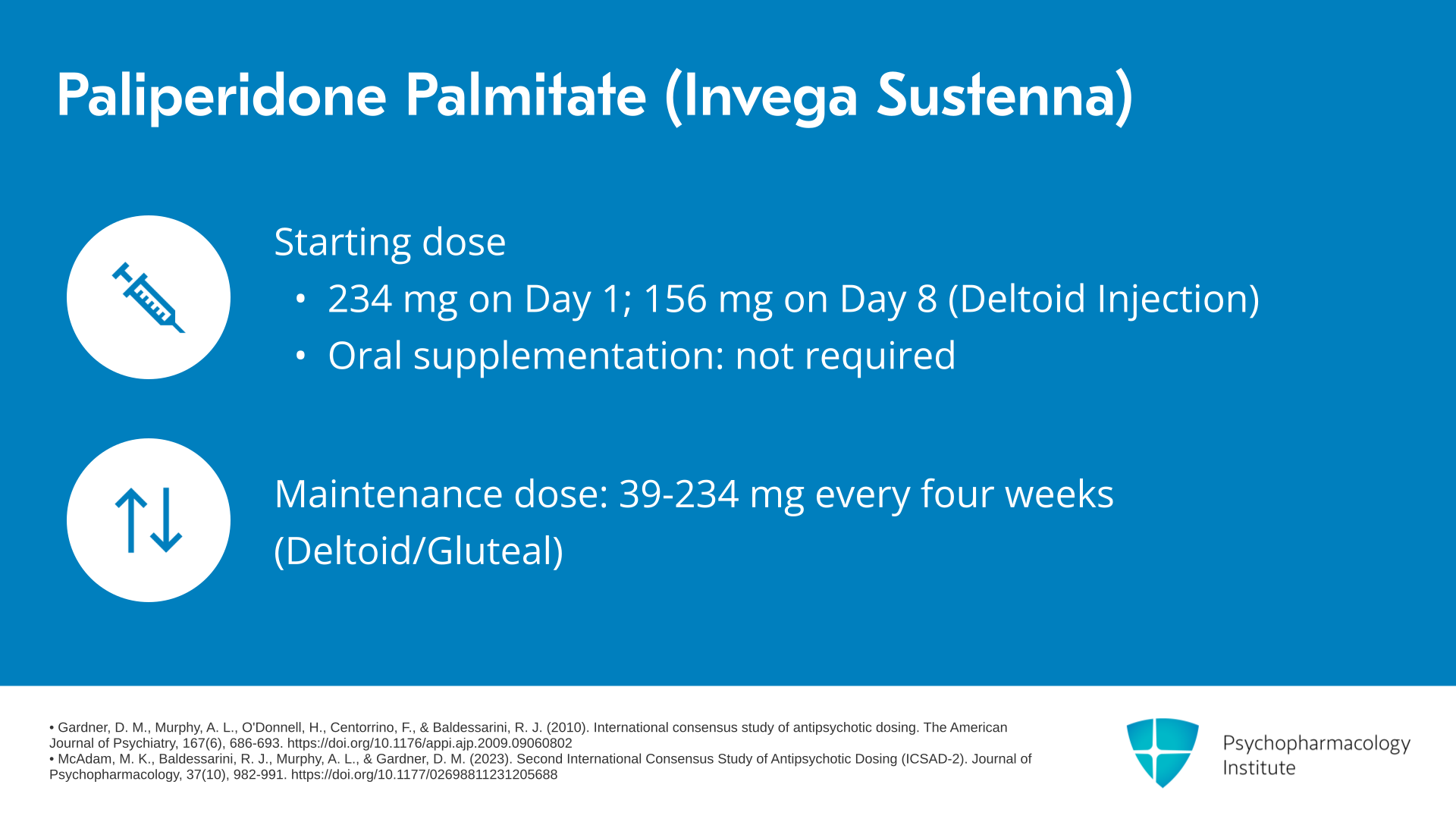
There are multiple formulations of paliperidone palmitate. We’ll begin by talking about Invega Sustenna, the first form. The typical starting dose for Invega Sustenna is 234 mg given on day 1 and then 156 mg given on day 8 by deltoid injection. In contrast to other agents, oral supplementation is not required. And the typical maintenance doses for Invega Sustenna are 39, 78, 117, 156 or 234 mg given every four weeks by deltoid or gluteal injection.
References:
- Gardner, D. M., Murphy, A. L., O'Donnell, H., Centorrino, F., & Baldessarini, R. J. (2010). International consensus study of antipsychotic dosing. The American Journal of Psychiatry, 167(6), 686-693. https://doi.org/10.1176/appi.ajp.2009.09060802
- McAdam, M. K., Baldessarini, R. J., Murphy, A. L., & Gardner, D. M. (2023). Second International Consensus Study of Antipsychotic Dosing (ICSAD-2). Journal of Psychopharmacology, 37(10), 982-991. https://doi.org/10.1177/02698811231205688
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 3 of 19
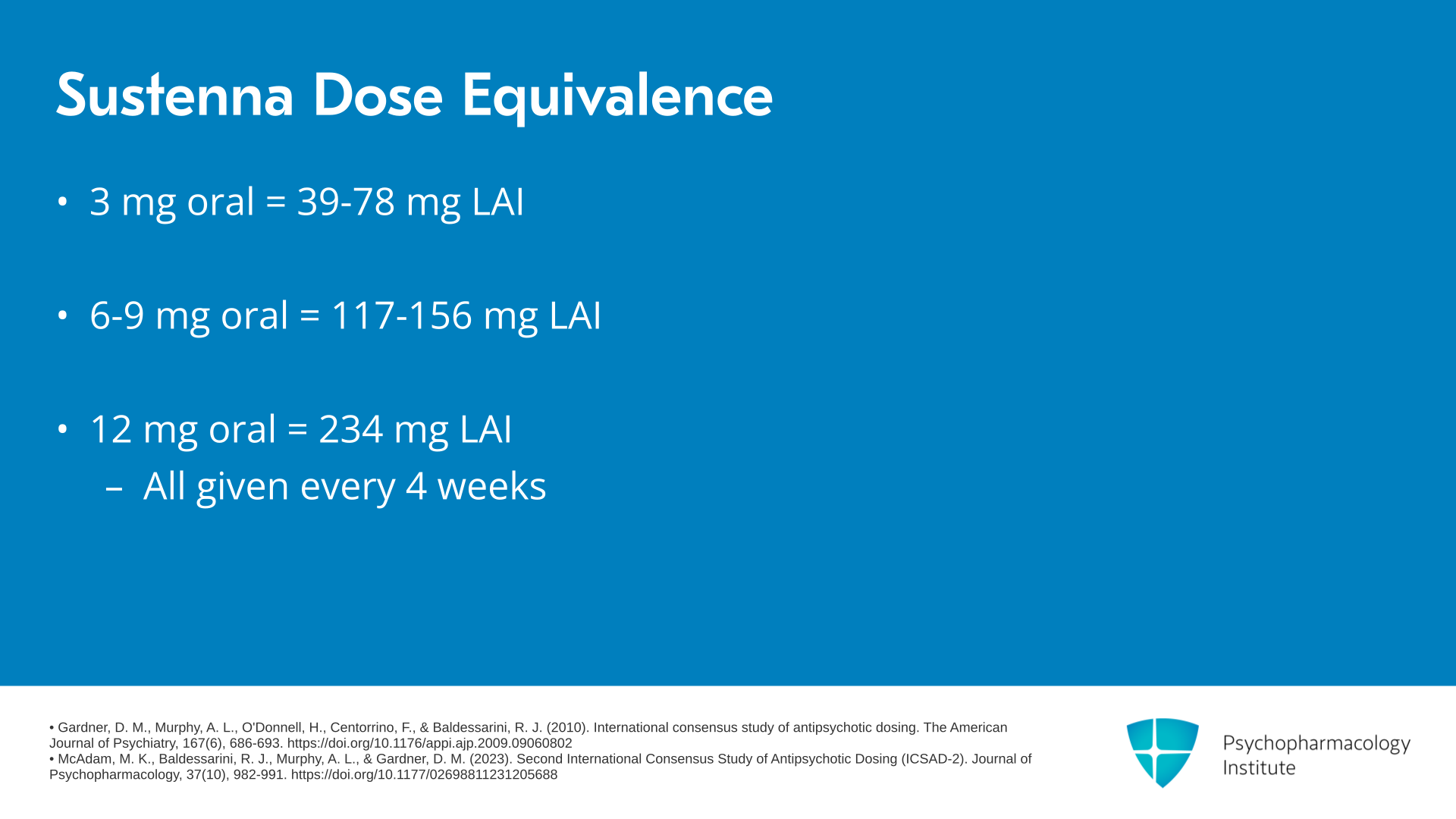
In terms of dose equivalency, 3 mg of oral paliperidone corresponds to between 39 and 78 mg of the LAI, 6 mg oral corresponds to 117 mg of the LAI, 9 mg corresponds to 156 mg of the LAI, and 12 mg oral corresponds to 234 mg of the LAI, all given every four weeks.
References:
- Gardner, D. M., Murphy, A. L., O'Donnell, H., Centorrino, F., & Baldessarini, R. J. (2010). International consensus study of antipsychotic dosing. The American Journal of Psychiatry, 167(6), 686-693. https://doi.org/10.1176/appi.ajp.2009.09060802
- McAdam, M. K., Baldessarini, R. J., Murphy, A. L., & Gardner, D. M. (2023). Second International Consensus Study of Antipsychotic Dosing (ICSAD-2). Journal of Psychopharmacology, 37(10), 982-991. https://doi.org/10.1177/02698811231205688
Slide 4 of 19
If a patient has a delayed or missed dose of at least six weeks, they can be given their maintenance dose on day 1. If the delay or the missed dose is greater than six months, then the patient should be reloaded as per the package insert.
References:
- Gardner, D. M., Murphy, A. L., O'Donnell, H., Centorrino, F., & Baldessarini, R. J. (2010). International consensus study of antipsychotic dosing. The American Journal of Psychiatry, 167(6), 686-693. https://doi.org/10.1176/appi.ajp.2009.09060802
- McAdam, M. K., Baldessarini, R. J., Murphy, A. L., & Gardner, D. M. (2023). Second International Consensus Study of Antipsychotic Dosing (ICSAD-2). Journal of Psychopharmacology, 37(10), 982-991. https://doi.org/10.1177/02698811231205688
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 5 of 19
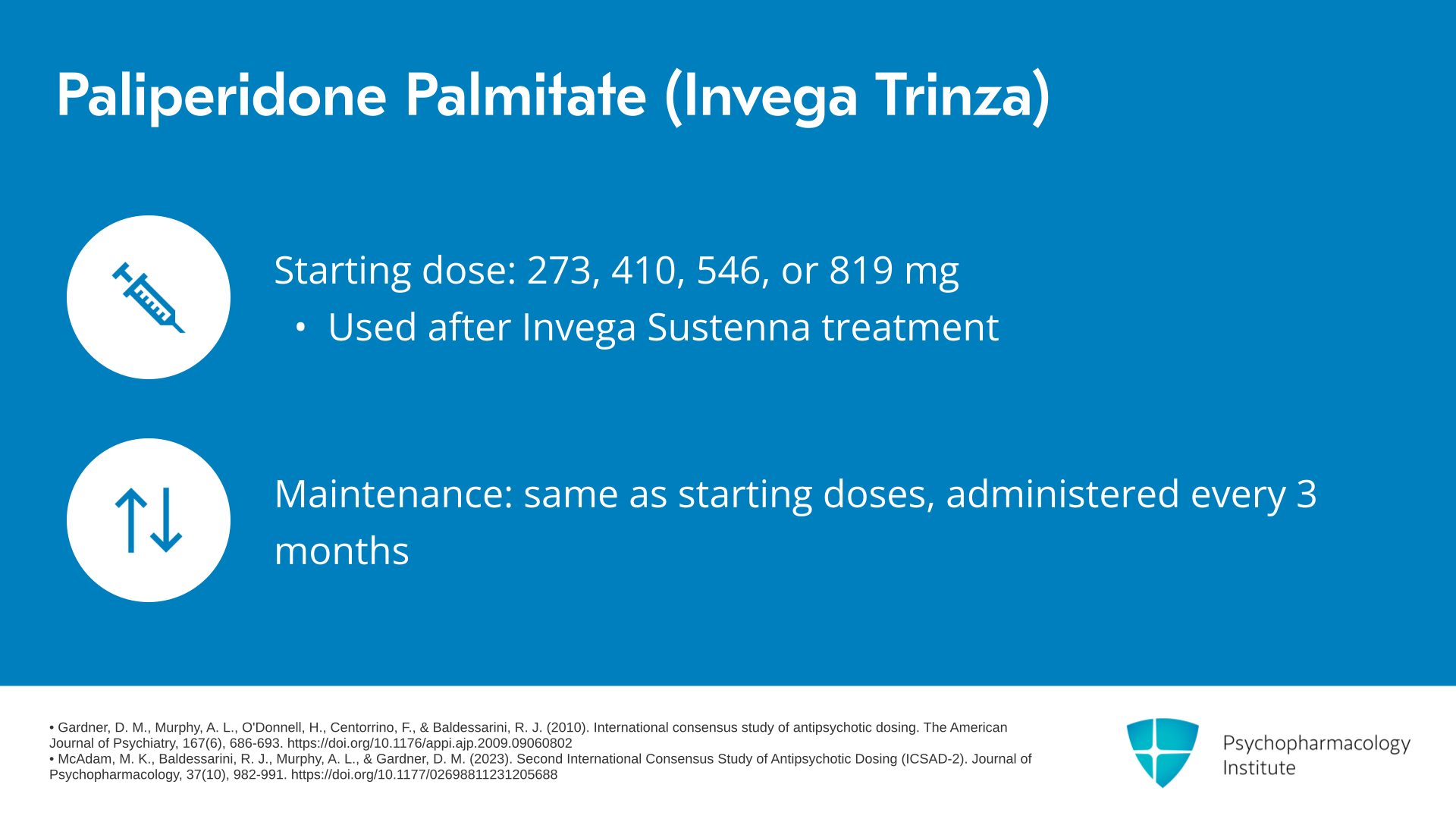
The next formulation of paliperidone palmitate is Invega Trinza. Typical starting doses for Trinza are 273, 410, 546 or 819 mg. This agent would be used in patients who have already been treated with a monthly formulation Invega Sustenna. And the typical maintenance doses are the same as the starting doses – 273, 410, 546 or 819 mg.
References:
- Gardner, D. M., Murphy, A. L., O'Donnell, H., Centorrino, F., & Baldessarini, R. J. (2010). International consensus study of antipsychotic dosing. The American Journal of Psychiatry, 167(6), 686-693. https://doi.org/10.1176/appi.ajp.2009.09060802
- McAdam, M. K., Baldessarini, R. J., Murphy, A. L., & Gardner, D. M. (2023). Second International Consensus Study of Antipsychotic Dosing (ICSAD-2). Journal of Psychopharmacology, 37(10), 982-991. https://doi.org/10.1177/02698811231205688
Slide 6 of 19
In terms of dose equivalence, patients who are getting 78 mg of Sustenna per month should receive 273 mg of Trinza every three months, 117 mg of Sustenna corresponds to 410 mg of Trinza, 156 mg of Sustenna corresponds to 546 mg of Trinza, and 234 mg of Sustenna corresponds to 819 mg of Trinza again which is given every 3 months.
References:
- Gardner, D. M., Murphy, A. L., O'Donnell, H., Centorrino, F., & Baldessarini, R. J. (2010). International consensus study of antipsychotic dosing. The American Journal of Psychiatry, 167(6), 686-693. https://doi.org/10.1176/appi.ajp.2009.09060802
- McAdam, M. K., Baldessarini, R. J., Murphy, A. L., & Gardner, D. M. (2023). Second International Consensus Study of Antipsychotic Dosing (ICSAD-2). Journal of Psychopharmacology, 37(10), 982-991. https://doi.org/10.1177/02698811231205688
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 7 of 19
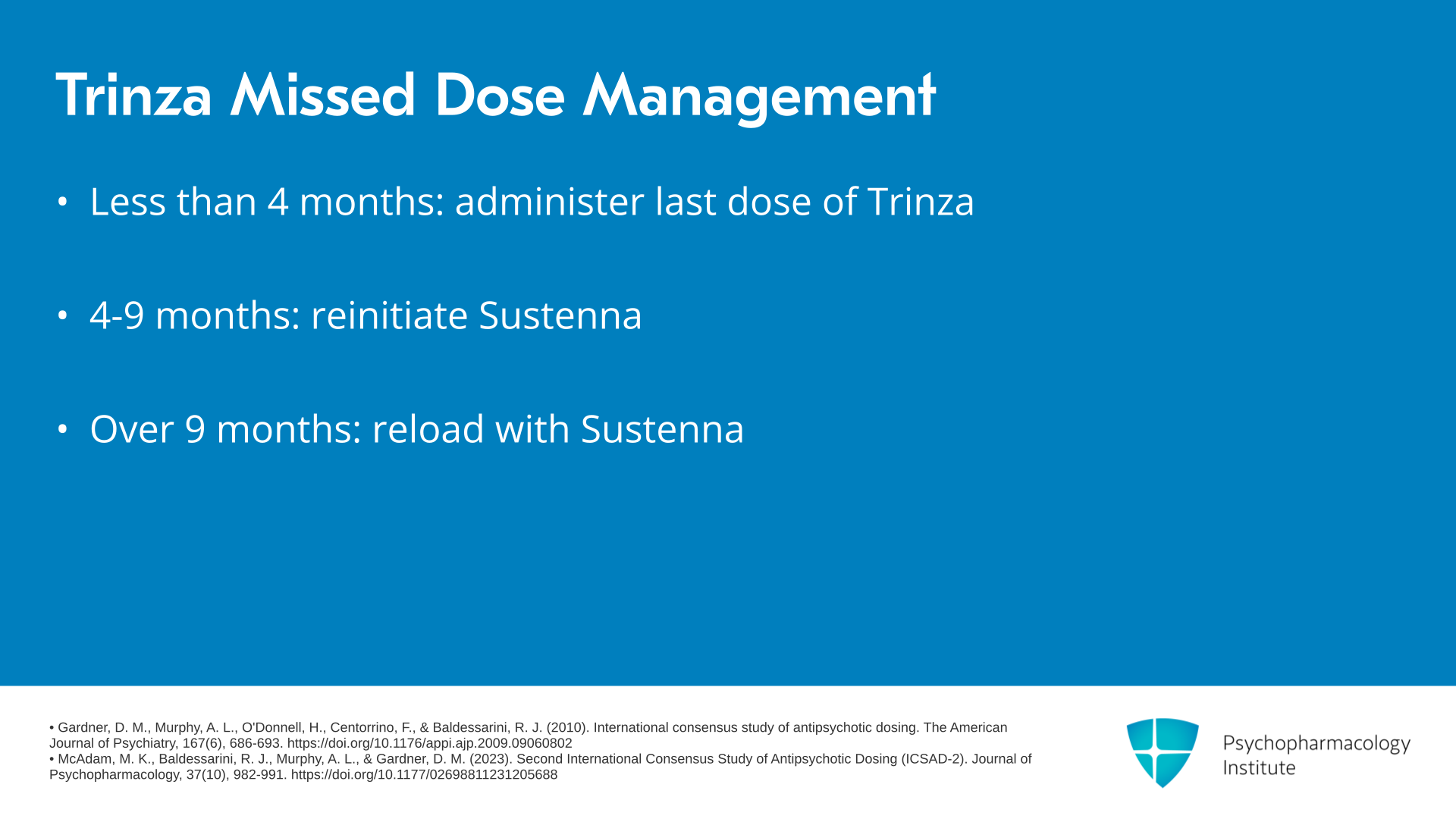
What happens if patients miss doses? If the delay is less than four months, they should be administered their last dose of Trinza. If the missed dose period is between four and nine months, they should be reinitiated with Invega Sustenna. And if the delay is greater than nine months, they should be reloaded with Invega Sustenna.
References:
- Gardner, D. M., Murphy, A. L., O'Donnell, H., Centorrino, F., & Baldessarini, R. J. (2010). International consensus study of antipsychotic dosing. The American Journal of Psychiatry, 167(6), 686-693. https://doi.org/10.1176/appi.ajp.2009.09060802
- McAdam, M. K., Baldessarini, R. J., Murphy, A. L., & Gardner, D. M. (2023). Second International Consensus Study of Antipsychotic Dosing (ICSAD-2). Journal of Psychopharmacology, 37(10), 982-991. https://doi.org/10.1177/02698811231205688
Slide 8 of 19
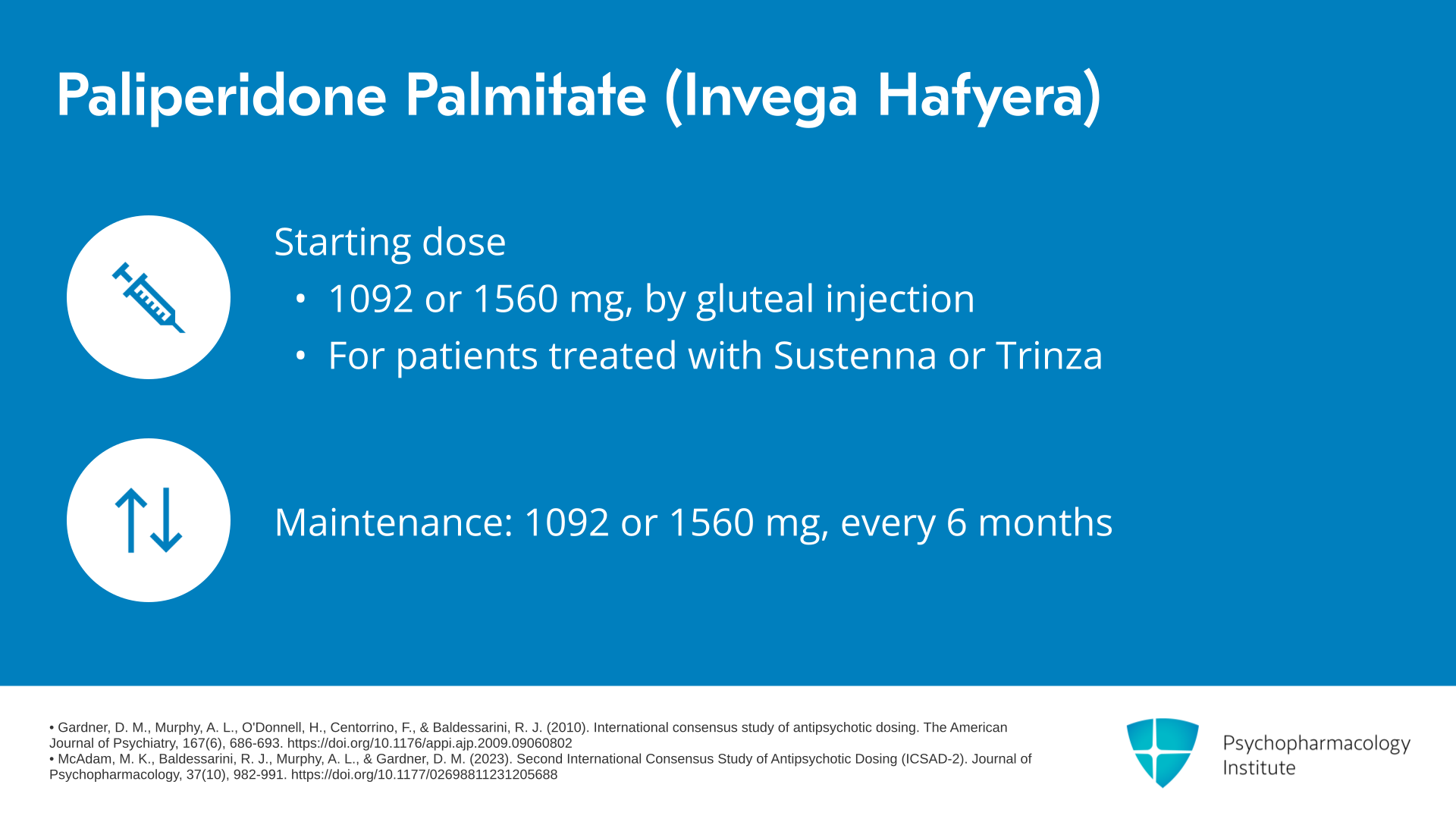
And then our final paliperidone palmitate formulation is Invega Hafyera which is an every-six-month formulation. The typical starting doses for Invega Hafyera are 1092 and 1560 mg every six months given by gluteal injection. This agent would be used in patients who are already treated with either Invega Sustenna or Invega Trinza. Again, the starting doses and the maintenance doses are the same, 1092 or 1560 mg every six months.
References:
- Gardner, D. M., Murphy, A. L., O'Donnell, H., Centorrino, F., & Baldessarini, R. J. (2010). International consensus study of antipsychotic dosing. The American Journal of Psychiatry, 167(6), 686-693. https://doi.org/10.1176/appi.ajp.2009.09060802
- McAdam, M. K., Baldessarini, R. J., Murphy, A. L., & Gardner, D. M. (2023). Second International Consensus Study of Antipsychotic Dosing (ICSAD-2). Journal of Psychopharmacology, 37(10), 982-991. https://doi.org/10.1177/02698811231205688
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 9 of 19
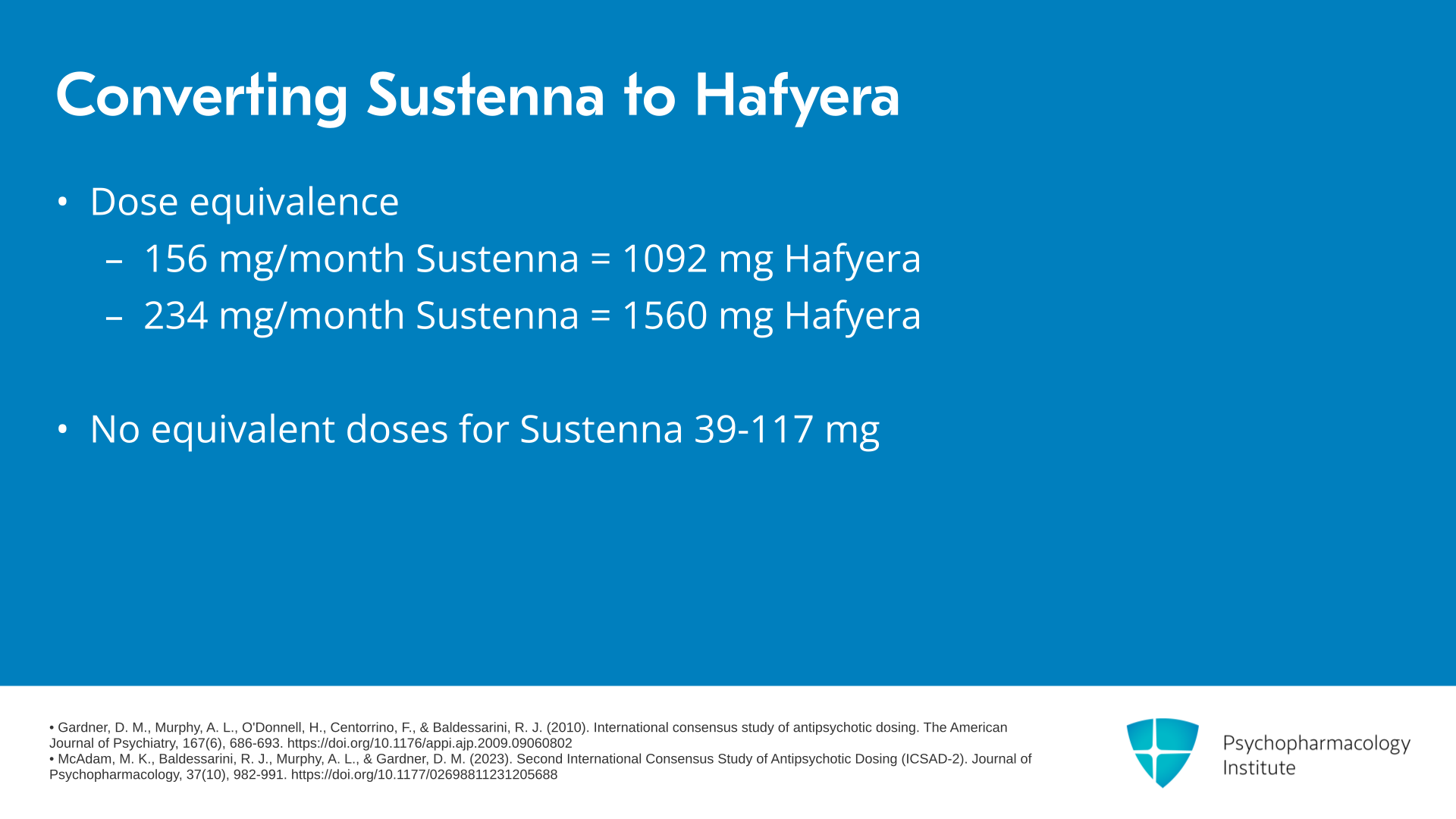
What is the dose equivalence here? Well, if we’re talking about Invega Sustenna, 156 mg per month of Sustenna corresponds to 1092 mg of Hafyera and 234 mg per month of Sustenna corresponds to 1560 mg of Hafyera. Importantly, there are no equivalent doses for the lower doses of Invega Sustenna, namely 39, 78 or 117 mg monthly.
References:
- Gardner, D. M., Murphy, A. L., O'Donnell, H., Centorrino, F., & Baldessarini, R. J. (2010). International consensus study of antipsychotic dosing. The American Journal of Psychiatry, 167(6), 686-693. https://doi.org/10.1176/appi.ajp.2009.09060802
- McAdam, M. K., Baldessarini, R. J., Murphy, A. L., & Gardner, D. M. (2023). Second International Consensus Study of Antipsychotic Dosing (ICSAD-2). Journal of Psychopharmacology, 37(10), 982-991. https://doi.org/10.1177/02698811231205688
Slide 10 of 19
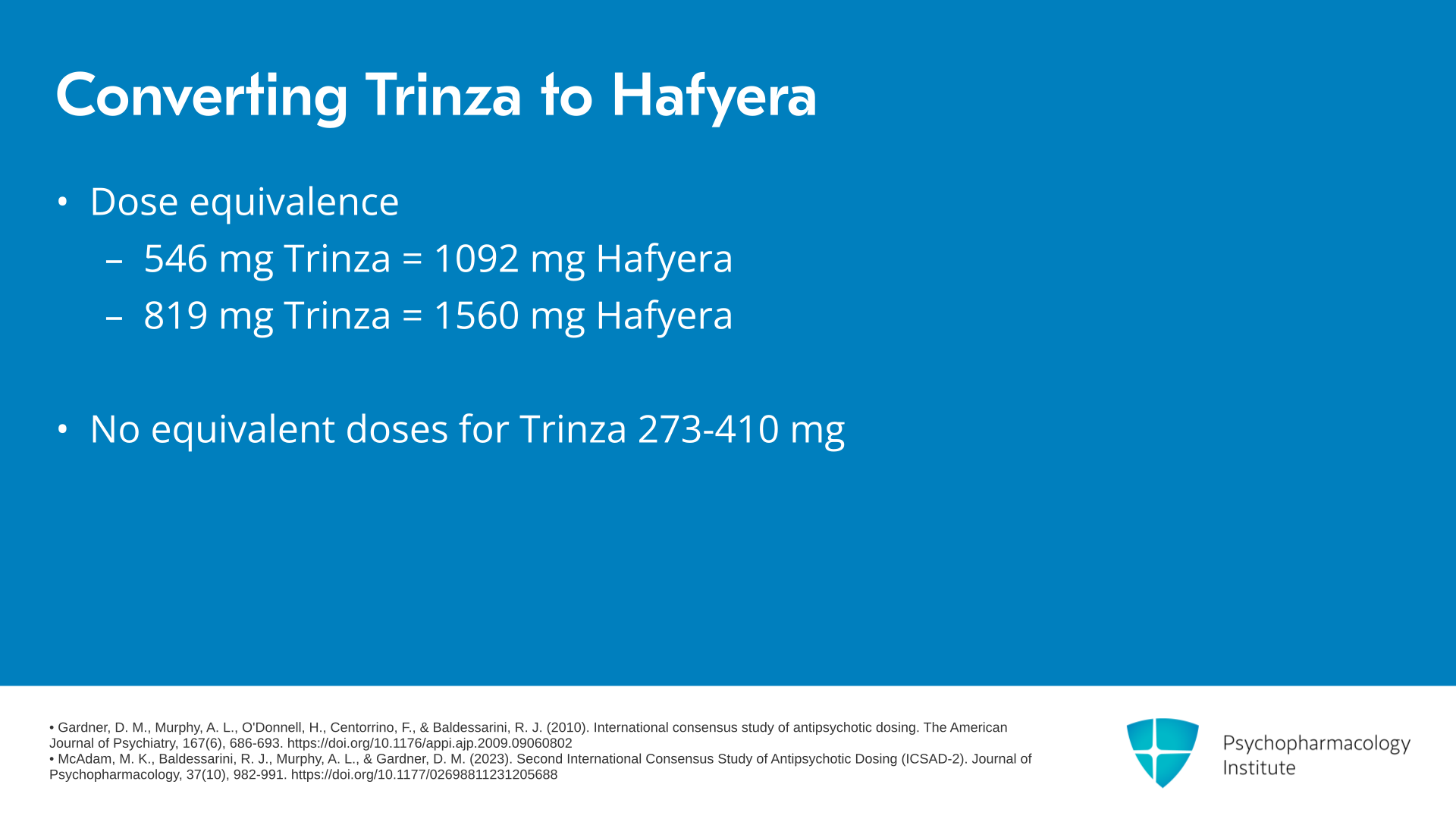
What about conversion dose equivalence between Invega Trinza and Hafyera? 546 mg of Trinza corresponds to 1092 of Hafyera and 819 mg of Trinza corresponds to 1560 mg of Hafyera. And similarly, there are no equivalent doses for the lower doses of Trinza, namely the 273 or 410 mg doses of Trinza.
References:
- Gardner, D. M., Murphy, A. L., O'Donnell, H., Centorrino, F., & Baldessarini, R. J. (2010). International consensus study of antipsychotic dosing. The American Journal of Psychiatry, 167(6), 686-693. https://doi.org/10.1176/appi.ajp.2009.09060802
- McAdam, M. K., Baldessarini, R. J., Murphy, A. L., & Gardner, D. M. (2023). Second International Consensus Study of Antipsychotic Dosing (ICSAD-2). Journal of Psychopharmacology, 37(10), 982-991. https://doi.org/10.1177/02698811231205688
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 11 of 19
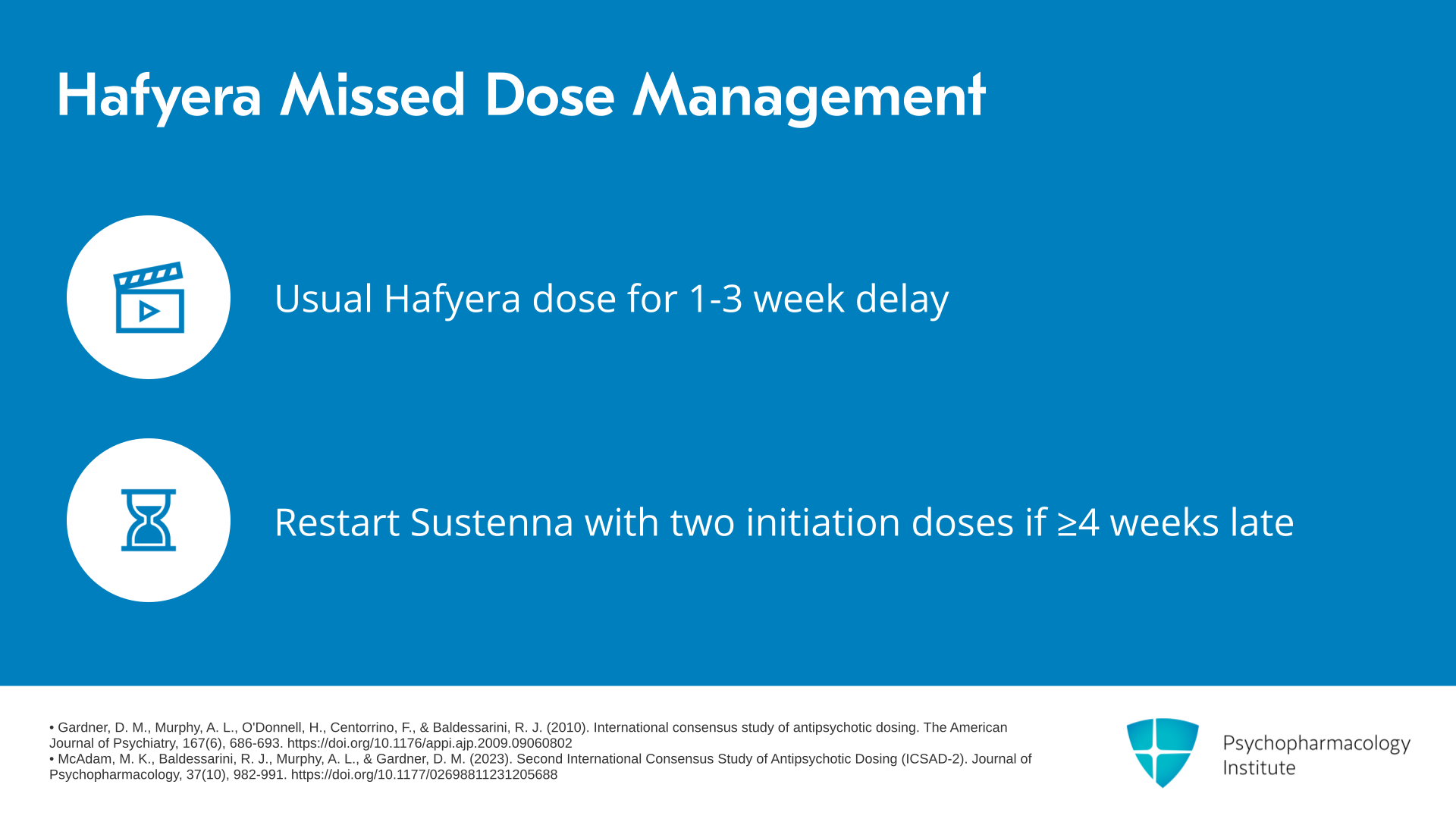
If a delay in injection is between one to three weeks, the usual dose of Hafyera can be given. By contrast, we would want to restart Invega Sustenna with two initiation doses if the patient is delayed by at least four weeks or more for their Hafyera injection.
References:
- Gardner, D. M., Murphy, A. L., O'Donnell, H., Centorrino, F., & Baldessarini, R. J. (2010). International consensus study of antipsychotic dosing. The American Journal of Psychiatry, 167(6), 686-693. https://doi.org/10.1176/appi.ajp.2009.09060802
- McAdam, M. K., Baldessarini, R. J., Murphy, A. L., & Gardner, D. M. (2023). Second International Consensus Study of Antipsychotic Dosing (ICSAD-2). Journal of Psychopharmacology, 37(10), 982-991. https://doi.org/10.1177/02698811231205688
Slide 12 of 19
Now, we’ll talk about two different formulations of risperidone. The first is risperidone or Perseris. The typical starting dose for Perseris is 90 or 120 mg injected subcutaneously every month. The injection is either in the abdomen or in the back of the upper arm. Oral supplementation is not required. The typical maintenance dose is the same as the starting dose, 90 to 120 mg monthly.
References:
- Gardner, D. M., Murphy, A. L., O'Donnell, H., Centorrino, F., & Baldessarini, R. J. (2010). International consensus study of antipsychotic dosing. The American Journal of Psychiatry, 167(6), 686-693. https://doi.org/10.1176/appi.ajp.2009.09060802
- McAdam, M. K., Baldessarini, R. J., Murphy, A. L., & Gardner, D. M. (2023). Second International Consensus Study of Antipsychotic Dosing (ICSAD-2). Journal of Psychopharmacology, 37(10), 982-991. https://doi.org/10.1177/02698811231205688
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 13 of 19
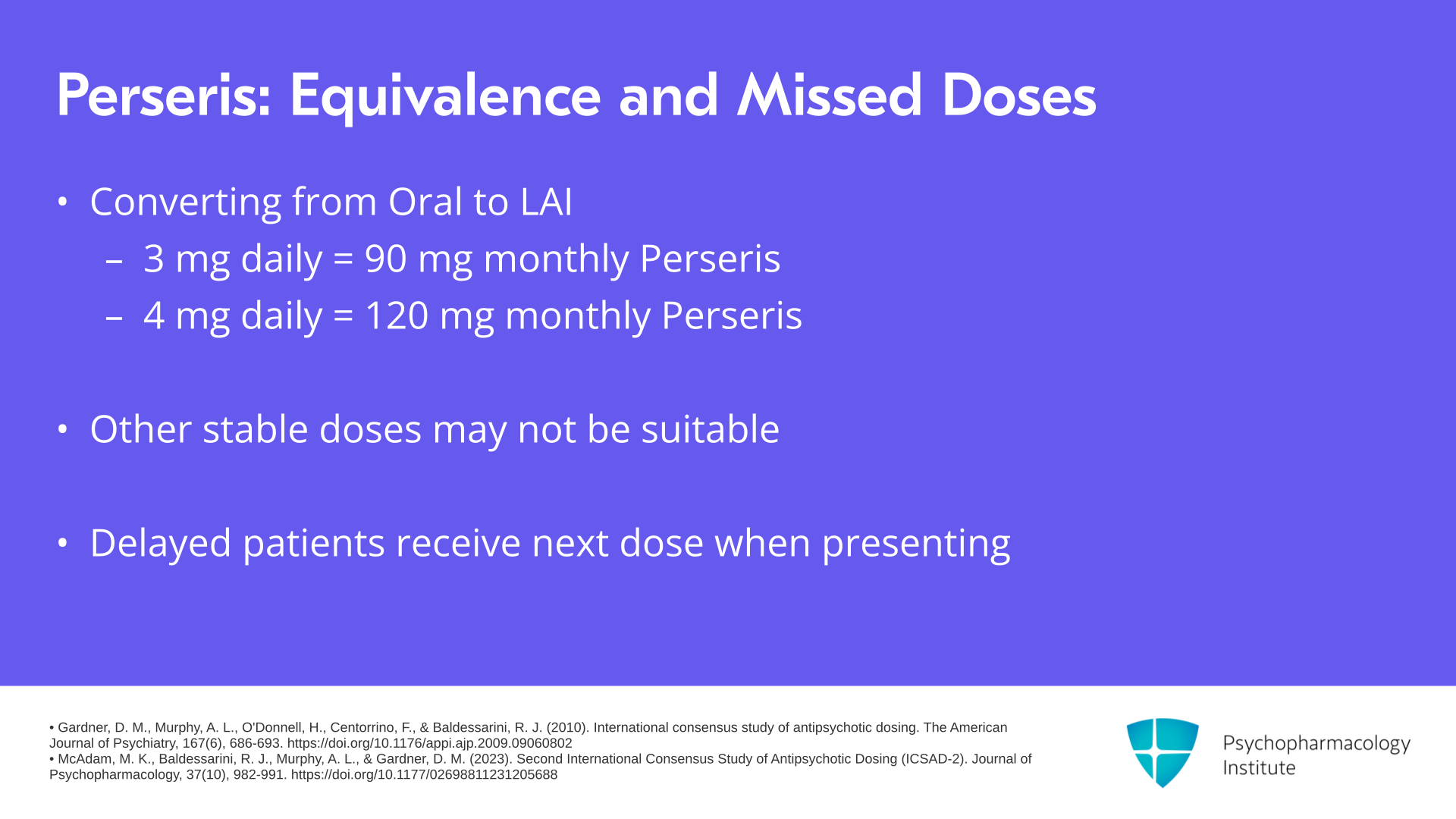
When it comes to dose equivalence, 3 mg daily of oral risperidone corresponds to 90 mg monthly of Perseris. 4 mg daily of oral risperidone corresponds to 120 mg monthly of Perseris. Other stable doses of oral risperidone may not be candidates for Perseris based on the dose equivalence information. And if a patient is delayed, they should be given their next dose of Perseris whenever they present to the clinic.
References:
- Gardner, D. M., Murphy, A. L., O'Donnell, H., Centorrino, F., & Baldessarini, R. J. (2010). International consensus study of antipsychotic dosing. The American Journal of Psychiatry, 167(6), 686-693. https://doi.org/10.1176/appi.ajp.2009.09060802
- McAdam, M. K., Baldessarini, R. J., Murphy, A. L., & Gardner, D. M. (2023). Second International Consensus Study of Antipsychotic Dosing (ICSAD-2). Journal of Psychopharmacology, 37(10), 982-991. https://doi.org/10.1177/02698811231205688
Slide 14 of 19
Our other formulation is risperidone microspheres or Risperdal Consta. The typical starting dose for Risperdal Consta would be either 12.5, 25 most commonly, 37.5 or 50 mg given by deltoid or gluteal injection. Oral supplementation is continued for 21 days. And the typical maintenance dose for Risperdal Consta is 12.5 to 50 mg given every two weeks.
References:
- Gardner, D. M., Murphy, A. L., O'Donnell, H., Centorrino, F., & Baldessarini, R. J. (2010). International consensus study of antipsychotic dosing. The American Journal of Psychiatry, 167(6), 686-693. https://doi.org/10.1176/appi.ajp.2009.09060802
- McAdam, M. K., Baldessarini, R. J., Murphy, A. L., & Gardner, D. M. (2023). Second International Consensus Study of Antipsychotic Dosing (ICSAD-2). Journal of Psychopharmacology, 37(10), 982-991. https://doi.org/10.1177/02698811231205688
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 15 of 19
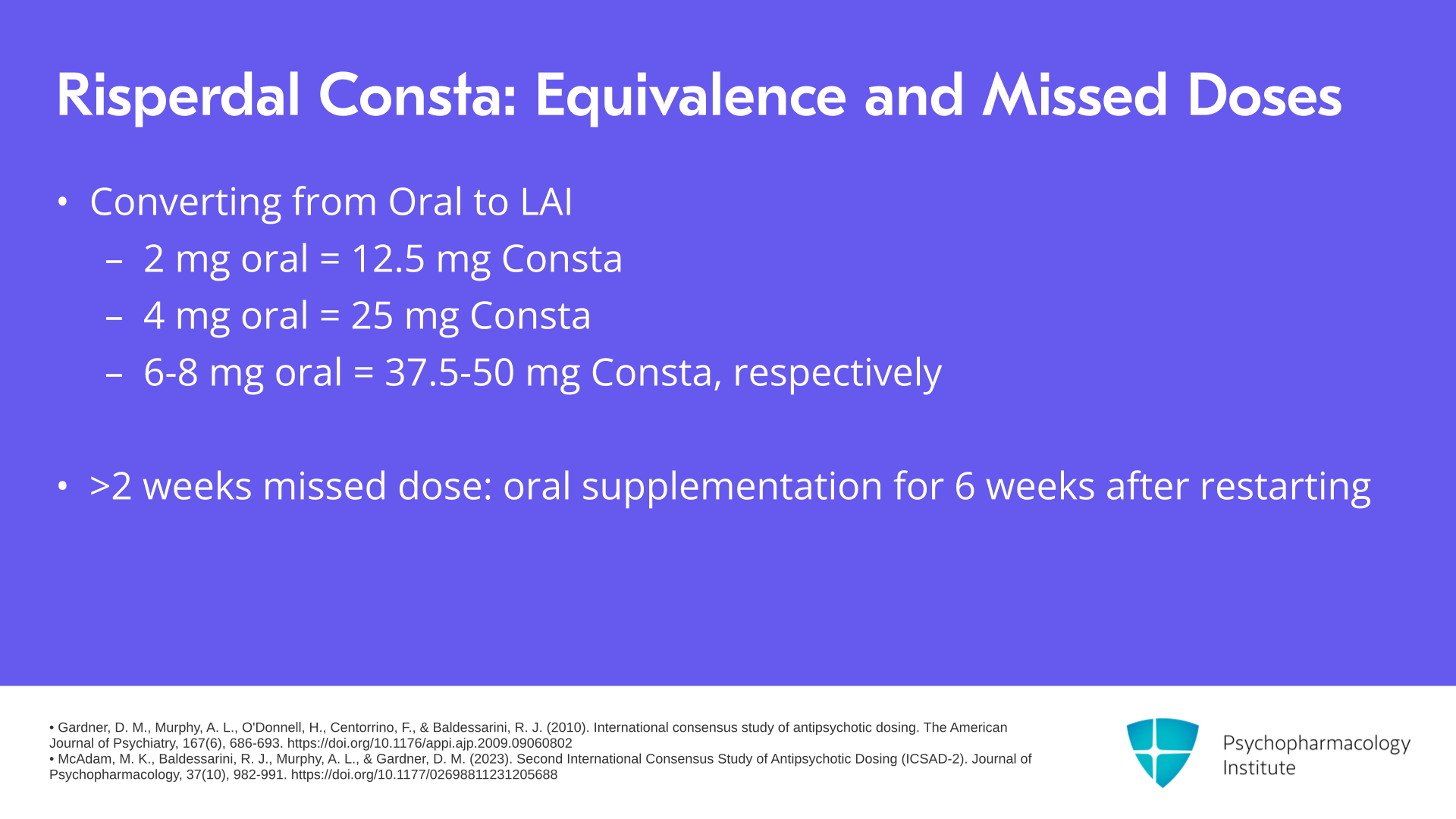
In terms of dose equivalence, 2 mg per day of oral risperidone corresponds to 12.5 mg of Consta, 4 mg a day oral corresponds to 25 mg Consta, 6 mg a day oral risperidone corresponds to 37.5 mg of Consta and 8 mg a day of oral risperidone corresponds to 50 mg of Consta, again all given every two weeks. If a patient misses a dose for more than two weeks, consider giving oral supplementation for six weeks after restarting.
References:
- Gardner, D. M., Murphy, A. L., O'Donnell, H., Centorrino, F., & Baldessarini, R. J. (2010). International consensus study of antipsychotic dosing. The American Journal of Psychiatry, 167(6), 686-693. https://doi.org/10.1176/appi.ajp.2009.09060802
- McAdam, M. K., Baldessarini, R. J., Murphy, A. L., & Gardner, D. M. (2023). Second International Consensus Study of Antipsychotic Dosing (ICSAD-2). Journal of Psychopharmacology, 37(10), 982-991. https://doi.org/10.1177/02698811231205688
Slide 16 of 19
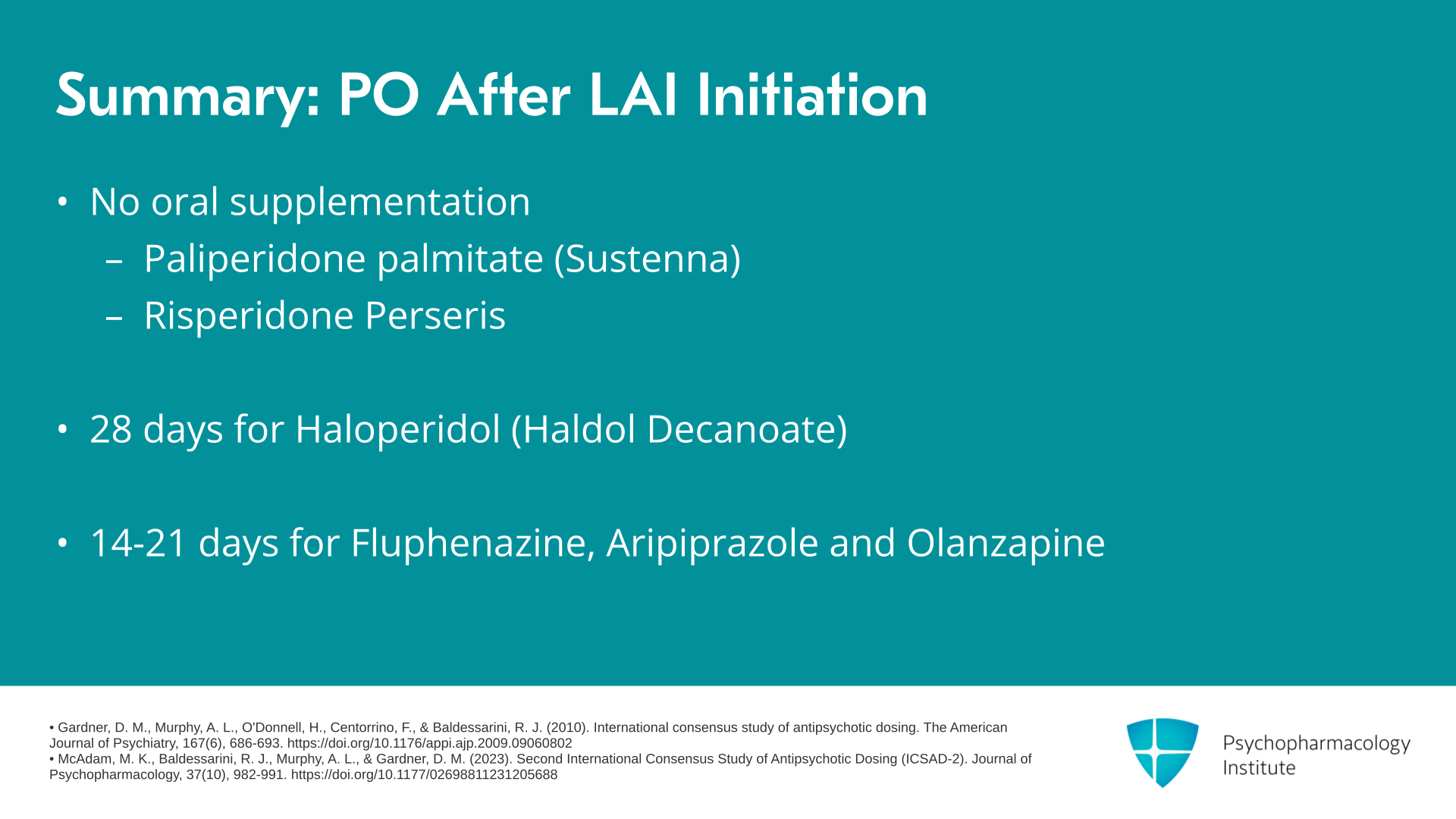
So as kind of a summary reference here, one of the important aspects of initiation is how long do I continue the oral antipsychotic medication after initiation of an LAI antipsychotic? Importantly, both paliperidone palmitate monthly and risperidone Perseris do not require any oral supplementation. For haloperidol, oral supplementation would be continued for 28 days. And for fluphenazine and the aripiprazole formulations and olanzapine, oral supplementation would be continued for 14 to 21 days.
References:
- Gardner, D. M., Murphy, A. L., O'Donnell, H., Centorrino, F., & Baldessarini, R. J. (2010). International consensus study of antipsychotic dosing. The American Journal of Psychiatry, 167(6), 686-693. https://doi.org/10.1176/appi.ajp.2009.09060802
- McAdam, M. K., Baldessarini, R. J., Murphy, A. L., & Gardner, D. M. (2023). Second International Consensus Study of Antipsychotic Dosing (ICSAD-2). Journal of Psychopharmacology, 37(10), 982-991. https://doi.org/10.1177/02698811231205688
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 17 of 19
So key points for this section: For paliperidone palmitate, typical starting dose, 234 mg on day 1, 156 mg on day 8 given as a deltoid injection. Oral supplementation not required. And the typical maintenance dosing is between 39 and 234 mg every four weeks. Every 3-month, that’s Trinza, and every 6-month, that’s Hafyera, formulations are also available for paliperidone palmitate.
Slide 18 of 19
For risperidone Perseris, the typical starting and maintenance dose is between 90 and 125 mg monthly given in the abdomen or back of the upper arm. Oral supplementation is not required.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.