Text version
KarXT Mechanism and Clinical Use
Today I want to share a Quick Take on a recent publication summarizing clinical trial data for KarXT using a single number: NNT, or number needed to treat.
KarXT is the recently approved antipsychotic combining xanomeline with trospium. The FDA approved it last September. What’s different here is the mechanism—cholinergic agonism. Trospium, the “T” in KarXT, is added only to reduce peripheral cholinergic side effects. The antipsychotic action comes from xanomeline, the “X.” And in case you’re wondering, “Kar” stands for Karuna, the company that developed it.
Let’s say a patient hears about this new medication and wants to try it. As clinicians, we need to know two key things: Is it effective? And is it safe?
Download PDF and other files
NNT as a Clinical Translation Tool
You could now simply go to each individual registration trial of xanomeline to extract the information. Or, and that’s why we are here together, you could simply take the publication by Leslie Citrome that summarizes the available information in a helpful way for you and your patients using the so-called NNT or number needed to treat.
Let’s take a short sidebar on what NNT means. If you’ve come across this metric in psychiatry, you likely know Citrome. He’s made it his mission to translate trial data for practicing clinicians, including NNT and its counterpart, NNH (number needed to harm).
Both are basically shorthands to make more concrete the expected benefits and risks for our pharmacological treatments. The NNT is simply a way to translate the efficacy of a medication as determined in the clinical trial, for example, into an intuitive number. It gives you one possible estimate of an effect size.
It’s a metric defined as the number of patients who need to be treated to achieve one favorable outcome, one more favorable outcome compared to placebo usually. The NNT answers the question that you as a clinician would ask: For this medication, how many patients in my office would I need to treat to have one of the desired outcomes?
It takes into account there is a placebo response and that no medication works 100% for all patients. The desired outcome can also be the prevention of an undesired outcome like death or stroke. Put differently, the NNT is a more intuitive and simple way to estimate how likely a treatment is going to help the patient in front of you.
Calculating NNT
Here’s how you calculate it:
- Take the absolute difference in outcome rates (absolute risk reduction, ARR).
- Calculate its reciprocal.
- Round up to the next whole number—no fractional patients!
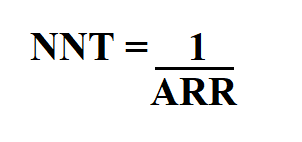
An NNT of:
- 1 = 100% efficacy (everyone benefits)
- 2 = one in two treated benefits
- Lower numbers = stronger efficacy
Generally, an NNT between 1–5 is considered very good. A single-digit NNT is considered clinically meaningful.
NNH follows the same structure but focuses on adverse events instead.
Download PDF and other files
Categorical Outcomes Are Required for NNT
One more point, the NNT requires a categorical or binary yes-no outcome like death or stroke. If the outcome variable is continuous like a psychopathology rating scale, we need to create a categorical outcome for the response versus a non-response. Like say a 30% reduction in psychopathology would count as a response.
Clinical trials usually provide such a measure and that is also the measure we will use for KarXT. So in this discussion here, the response to KarXT is a 30% reduction in psychopathology, not people becoming necessarily symptom free.
KarXT Trial Data: EMERGENT 1–3
The NNT for KarXT comes from three registration trials: EMERGENT 1, 2, and 3. Each was a five-week, placebo-controlled acute phase study with nearly identical design and outcome measures.
What’s the number? The pooled NNT for KarXT is 5. So, we’d need to treat five patients to see one additional response—defined here as a ≥30% reduction in psychopathology—after five weeks of treatment.
What about safety?
- NNH was generally >10 (which is good).
- Exception: nausea and vomiting had a lower NNH.
- However, the discontinuation rate due to GI side effects was only 49, suggesting these symptoms were often tolerated.
The study also includes LHH (likelihood to be helped or harmed), but I want you to remember one thing: KarXT has a single-digit NNT of 5.
Download PDF and other files
Clinical Implications of KarXT’s NNT
The single-digit NNT of 5 for KarXT indicates it’s an effective antipsychotic for acute phase treatment of psychosis. However, it’s important to note that not everyone will respond.
KarXT’s exact role in your treatment algorithm, when to use it, and for whom to use it, remains to be determined. Only time and more clinical experience will provide these answers.
Remember, while the NNT is a helpful metric, it doesn’t tell the whole story. It’s just one tool to help you make informed decisions about treatment options for your patients.
Abstract
Xanomeline and Trospium Chloride Versus Placebo for the Treatment of Schizophrenia: A Post Hoc Analysis of Number Needed to Treat, Number Needed to Harm, and Likelihood to Be Helped or Harmed
Leslie Citrome, Nichole M Neugebauer, Alicia A Meli & Judith Kando
Purpose: Describe xanomeline and trospium chloride efficacy and safety/tolerability for the treatment of schizophrenia using number needed to treat (NNT), number needed to harm (NNH), and likelihood to be helped or harmed (LHH).
Methods: Categorical data were extracted from the three 5-week, randomized, double blind, placebo controlled EMERGENT-1, EMERGENT-2, and EMERGENT-3 clinical trials of xanomeline/trospium in adults with schizophrenia experiencing acute psychosis. Efficacy was assessed using the Positive and Negative Syndrome Scale (PANSS), Clinical Global Impression-Severity (CGI-S), and categorical response criteria. Safety and tolerability were assessed using rates of discontinuation and treatment-emergent adverse events (TEAEs). NNT, NNH, and LHH values were calculated for each individual study as well as pooled.
Results: In data from the acute EMERGENT trials, NNT estimates were significant for xanomeline/trospium vs placebo for the pre-specified treatment response threshold of ≥30% reduction from baseline in PANSS total score at Week 5 (NNT=5 [95% CI, 4-8]). NNT estimates for response thresholds of ≥20% and ≥40% reduction from baseline in PANSS total score and ≥1- and ≥2-point decrease from baseline in CGI-S score were <10, indicating a clinically relevant therapeutic benefit of xanomeline/trospium over placebo. Estimates of NNH vs placebo for the most common TEAEs were >10, with the exception of nausea and vomiting; however, rates of discontinuations due to TEAEs of nausea, dyspepsia, or vomiting were low (NNH=49 [95% CI, 28-182]). LHH indicated an overall benefit of xanomeline/trospium vs placebo for all assessed outcomes. In indirect comparisons based on published data from trials of available antipsychotics approved for schizophrenia, xanomeline/trospium exhibited comparable or more robust NNT estimates vs placebo and was the least likely agent to be associated with weight gain or somnolence/sedation.
Conclusion: In the 5-week EMERGENT clinical trials, NNT, NNH, and LHH assessments demonstrated a favorable benefit-risk profile for xanomeline/trospium.
Trial registration: ClinicalTrials.gov identifiers: NCT03697252, NCT04659161, NCT04738123.
Keywords: KarXT; antipsychotic; number needed to harm; number needed to treat; schizophrenia; xanomeline and trospium chloride.
Download PDF and other files
Reference
Citrome, L.; Neugebauer, N.; Meli, A. & Kando, J. Xanomeline and Trospium Chloride Versus Placebo for the Treatment of Schizophrenia: A Post Hoc Analysis of Number Needed to Treat, Number Needed to Harm, and Likelihood to Be Helped or Harmed. (2025). Neuropsychiatr Dis Treat. 2025;21:761-773.



