Slides and Transcript
Slide 2 of 10
If you’re not familiar with ARFID or avoidant and restrictive food intake disorder, it is basically an upgrade from the feeding disorder of infancy and early childhood described in DSM-IV. For years, pediatricians have been dealing with what has been termed nonorganic failure to thrive in the older literature.
We know that many cases of ARFID have autism spectrum disorder symptoms in that they have major issues with food taste and textures that may drive their persistent failure to meet appropriate nutritional energy needs …
References:
- American Psychiatric Association. (2013). Feeding and eating disorders. In Diagnostic and Statistical Manual of Mental Disorders (5th ed.).
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 3 of 10
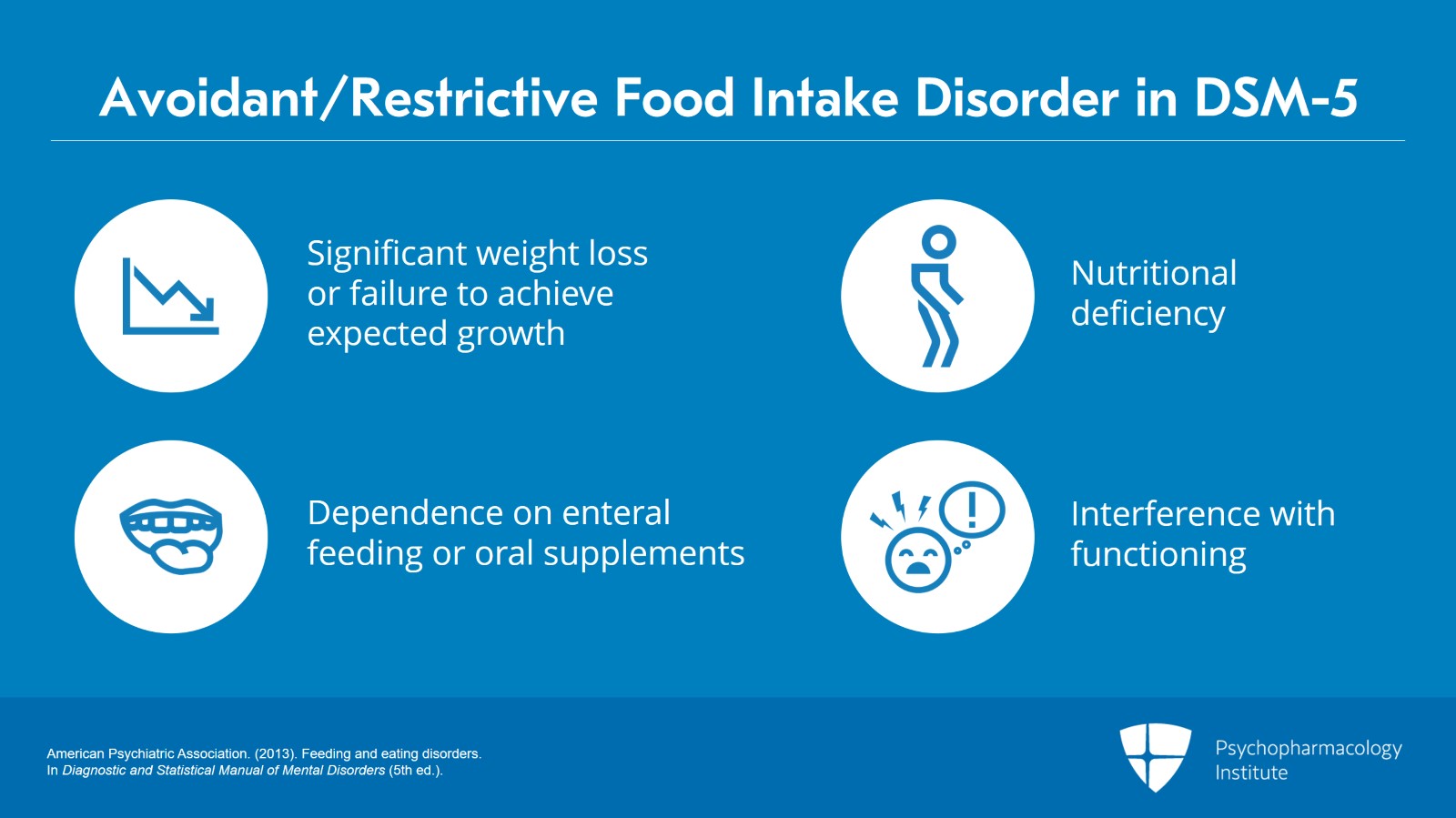
… and that leads to one of the following: Significant weight loss or failure to achieve expected growth, significant nutritional deficiency, dependence on enteral feeding or oral nutritional supplements and marked interference with psychosocial functioning.
References:
- American Psychiatric Association. (2013). Feeding and eating disorders. In Diagnostic and Statistical Manual of Mental Disorders (5th ed.).
Slide 4 of 10
And it’s not better explained by the lack of available food which has become known as food insecurity or a sanctioned cultural practice. It also does not occur during the course of anorexia nervosa or bulimia nervosa. And there is no evidence of a disturbance in the way in which one’s body, weight or shape is experienced. This is what distinguishes it from classic eating disorders.
Finally, ARFID symptoms are not attributable to a concurrent medical condition or mental disorder. And when it does occur, its severity exceeds that routinely associated with the condition or disorder and warrants additional clinical attention.
References:
- American Psychiatric Association. (2013). Feeding and eating disorders. In Diagnostic and Statistical Manual of Mental Disorders (5th ed.).
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 5 of 10
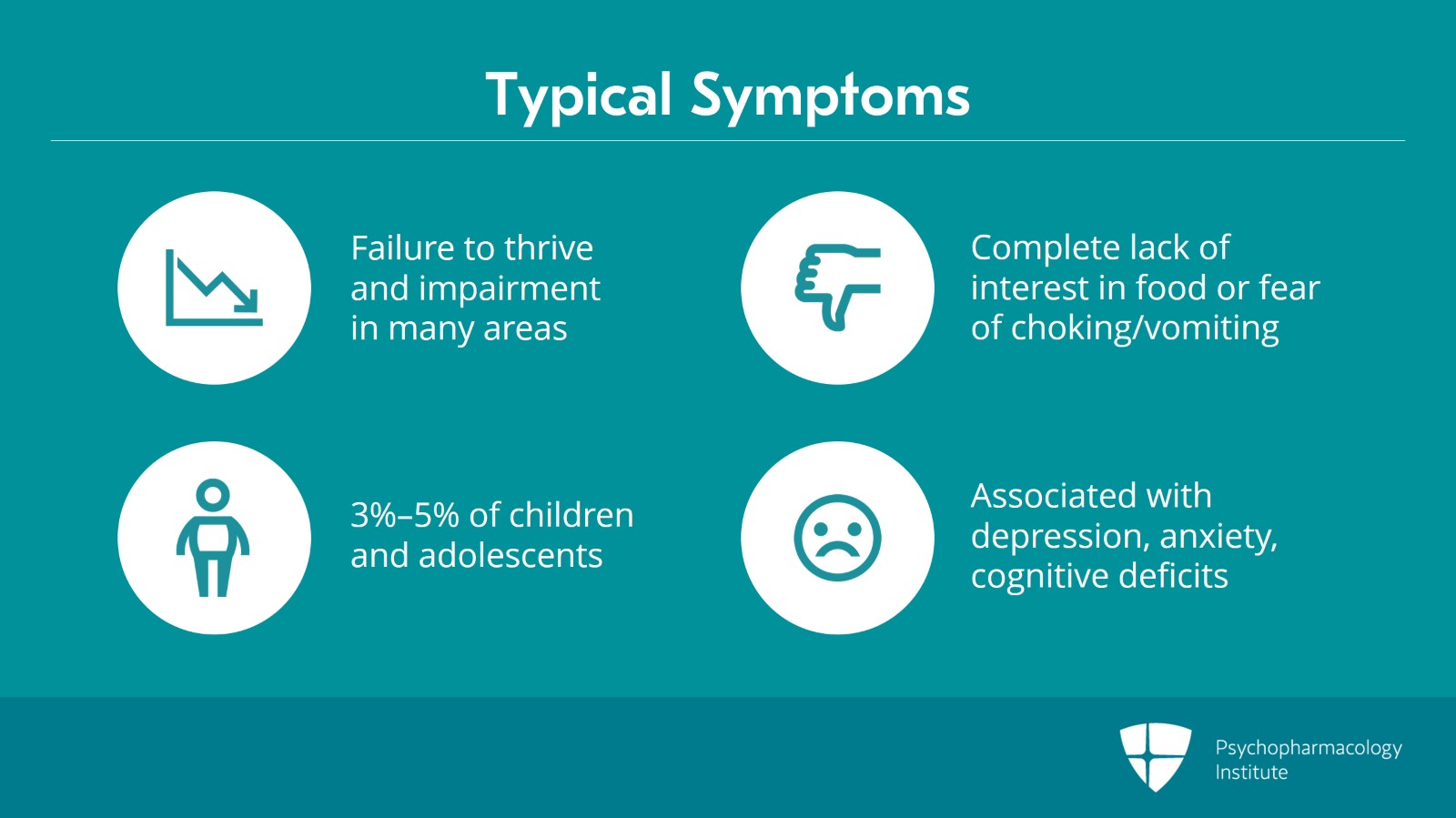
A typical patient with ARFID is a child or young adolescent who presents with failure to thrive in which there are varying degrees of impairment in many areas including physical, mental development, education and socialization as a result of the chronic starvation. The lack of interest in feeding or eating is not driven by body image distortion or fear of weight gain but rather by a complete lack of interest in eating or food and aversion to certain food textures or tastes or a fear of choking or vomiting usually due to a previous traumatic episode. ARFID occurs in about 3% to 5% of children and adolescents and there is often associated depression, anxiety and cognitive deficits.
Slide 6 of 10
There are no randomized controlled trials as of yet of agents for ARFID but there have been a number of case reports of adjunctive drug therapy.
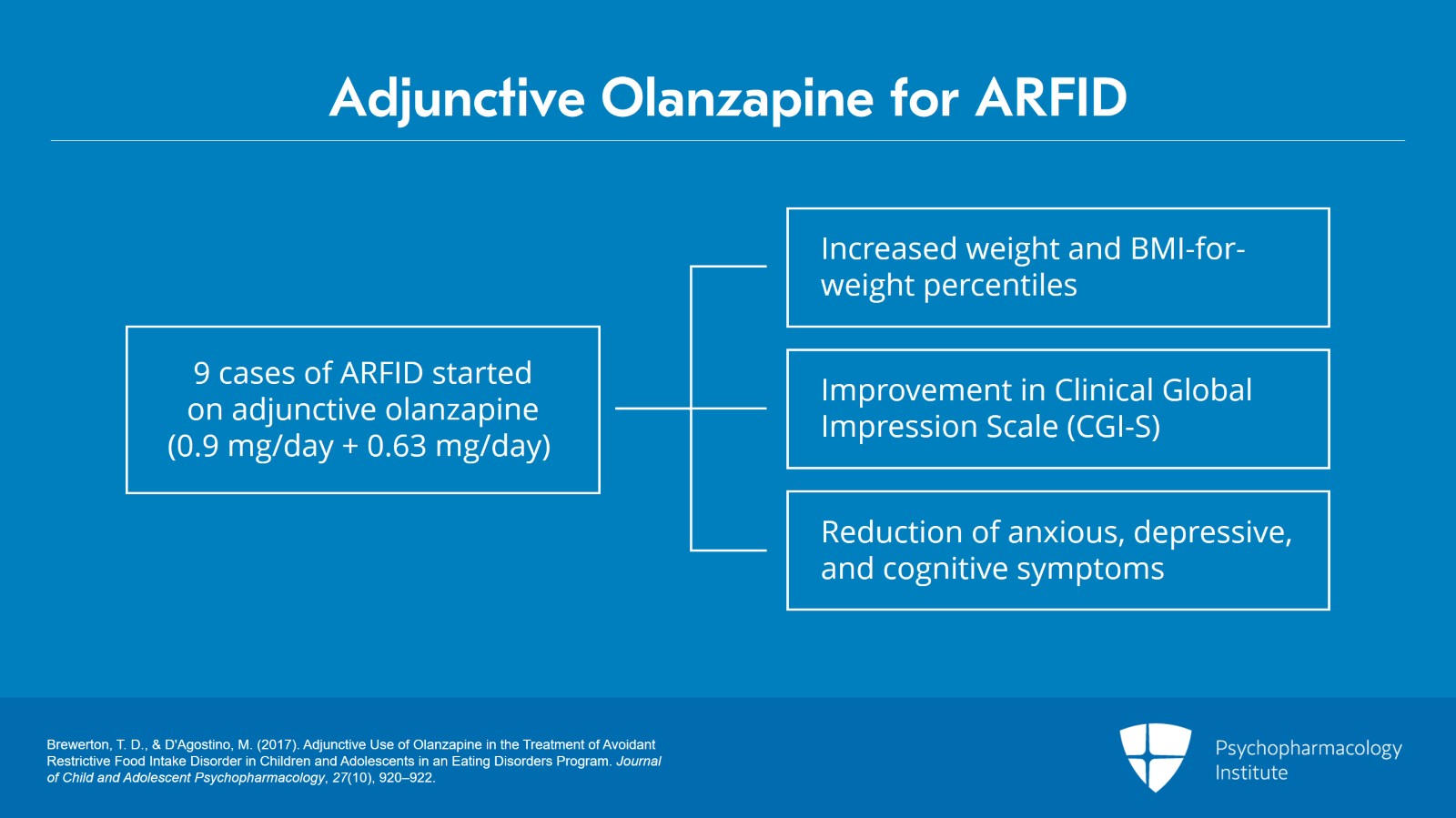
The treatment of ARFID is a multidisciplinary approach as it involves focus on behavioral treatment, sometimes occupational therapy. Meredith D’Agostino, a nurse practitioner, and I reported nine cases treated in an eating disorder program including eight females and one male with adjunctive olanzapine starting at a very, very low dose.
Average dose in this study was 0.9 mg. What you don’t want to do is overwhelm any patient or their family members with oversedation.
We found that there was significantly greater weight gain after introducing olanzapine in these individuals with ARFID. And they had marked improvements in their Clinical Global Impression Scale.
Not only did the weight increase and the feeding behavior improved but reduction of associated anxious, depressive and cognitive symptoms also very much improved. The same kind of story was reported a year later by Spettigue and associates regarding six cases.
References:
- Brewerton, T. D., & D'Agostino, M. (2017). Adjunctive Use of Olanzapine in the Treatment of Avoidant Restrictive Food Intake Disorder in Children and Adolescents in an Eating Disorders Program. Journal of Child and Adolescent Psychopharmacology, 27(10), 920–922.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 7 of 10
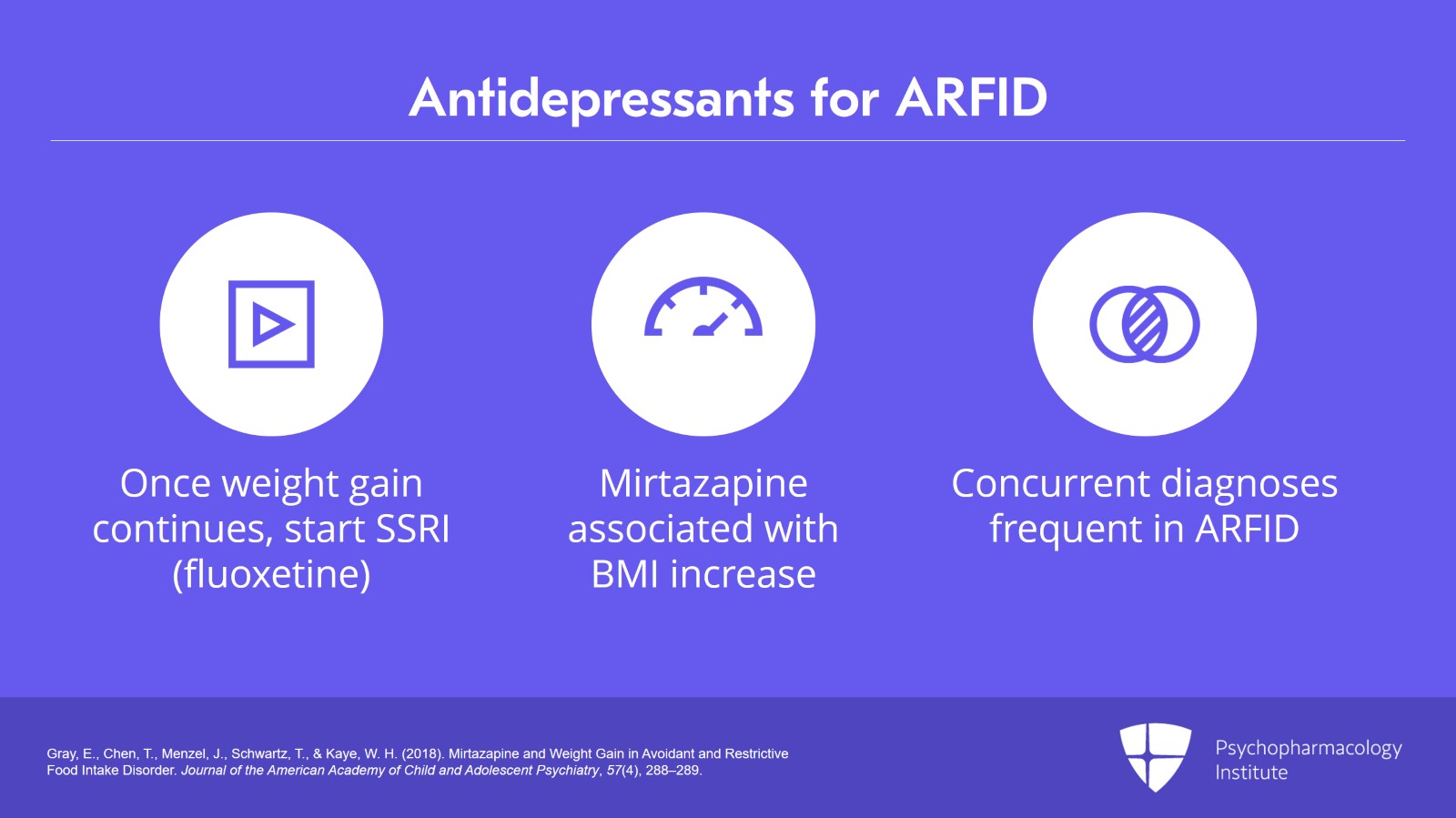
As is common practice, once the weight gain continues or approaches a normal weight, an SSRI, usually fluoxetine, is added to that. Gray and associates from the University of California at San Diego reported a retrospective chart review of 14 cases treated with mirtazapine. There were a number of concurrent diagnoses which tends to be the rule in feeding and eating disorders rather than the exception. They found that there was a significantly higher rate of increase in BMI.
References:
- Gray, E., Chen, T., Menzel, J., Schwartz, T., & Kaye, W. H. (2018). Mirtazapine and Weight Gain in Avoidant and Restrictive Food Intake Disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 57(4), 288–289.
Slide 8 of 10
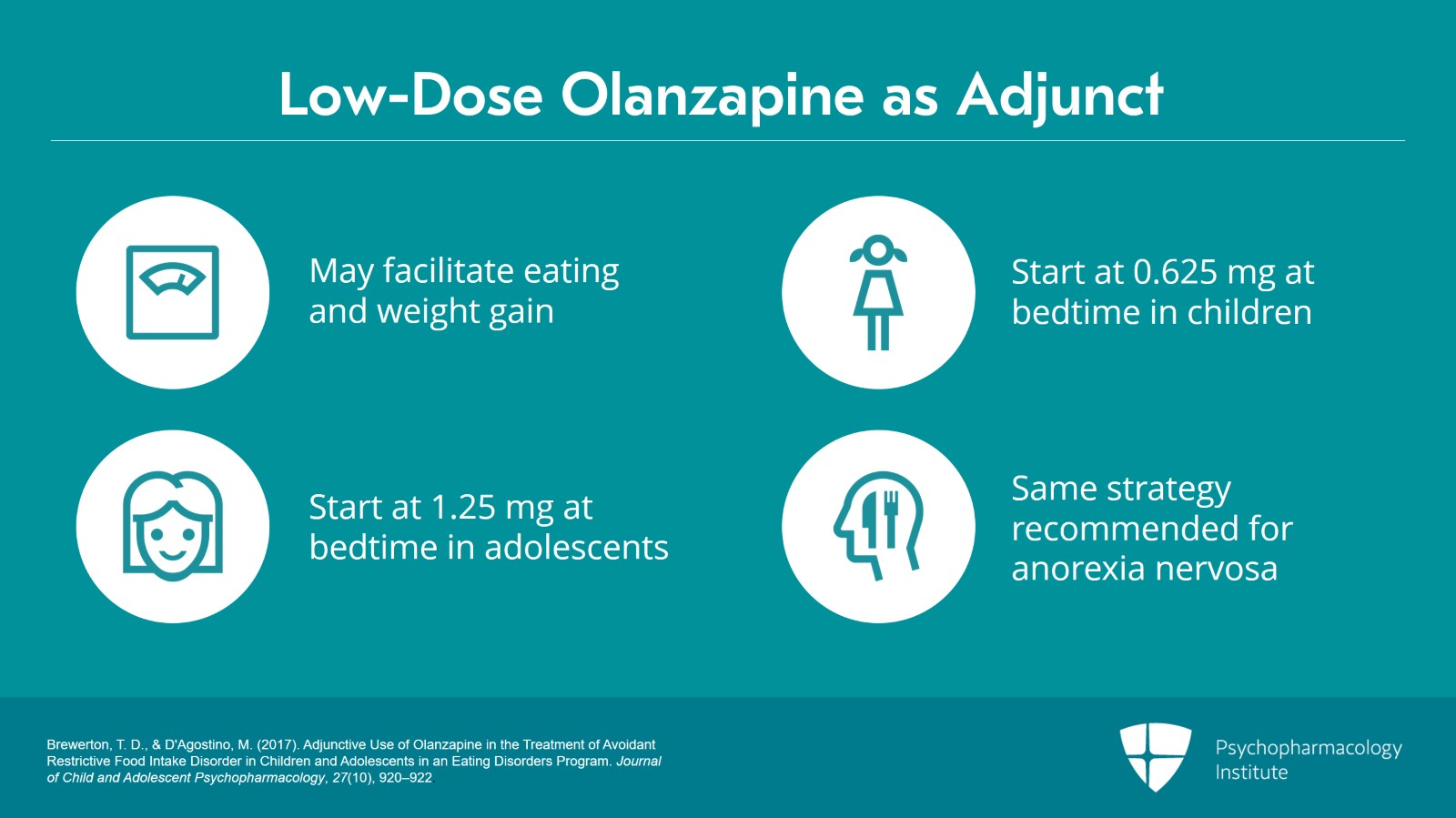
The judicious use of low-dose olanzapine when used as an adjunct to other treatment modalities may facilitate eating, weight gain and the reduction of anxious, depressive and cognitive symptoms.
It is highly recommended that super low doses be started, i.e., 0.625 mg at bedtime in children or 1.25 mg at bedtime in adolescents and then slowly titrating upward based on clinical response or side effects. But such low doses ensure the minimization of side effects.
And evidence suggests positive responses to very low doses in terms of weight gain and reduction of anxious, depressive and cognitive symptoms in conjunction with other therapies. As we’ll talk about later, this same strategy is recommended in the treatment of anorexia nervosa. Treatment continues indefinitely until normalization of eating behavior and developmental milestones occur or normalize.
References:
- Brewerton, T. D., & D'Agostino, M. (2017). Adjunctive Use of Olanzapine in the Treatment of Avoidant Restrictive Food Intake Disorder in Children and Adolescents in an Eating Disorders Program. Journal of Child and Adolescent Psychopharmacology, 27(10), 920–922.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 9 of 10
So the key points to this video. There are no randomized double-blind, placebo-controlled trials of any psychopharmacological agent for ARFID, only published retrospective case reports. The best evidence supports the use of very low dose adjunctive olanzapine. Mirtazapine starting at 7.5 mg per day has also been reported to be helpful for weight gain.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.