Slides and Transcript
Slide 1 of 8
With this, we're going to talk about the use of antipsychotics for bipolar disorder in pregnancy.
Slide 2 of 8
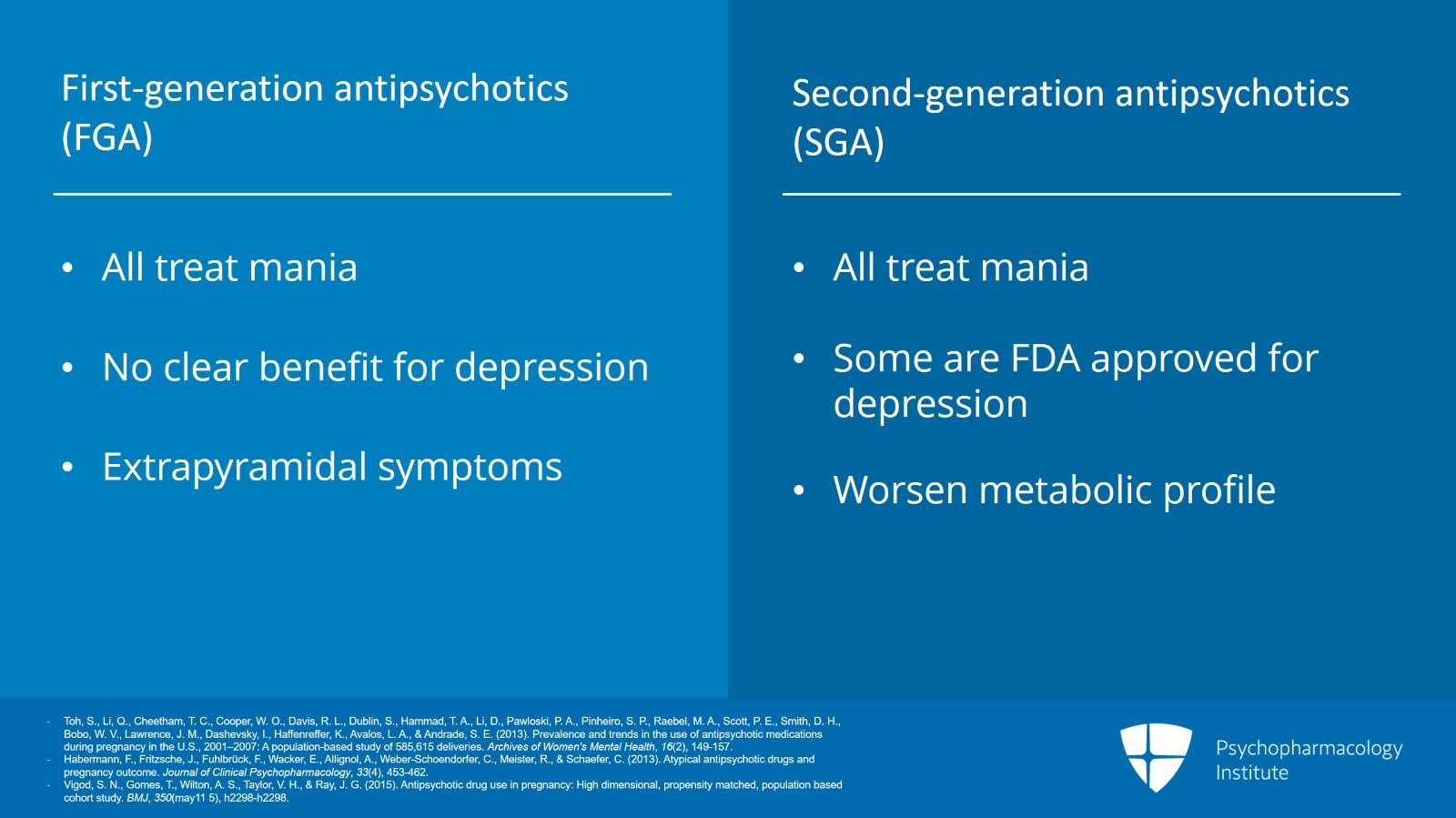
And a quick review, antipsychotics in bipolar disorder in general, we have the first generation otherwise known as the typicals. All of them treat mania. Not a clear benefit for depression. And classic adverse effects are the muscle movements or extrapyramidal symptoms. We also have the second-generation antipsychotics also known as the atypical antipsychotics. All these treat mania as well. Some of them have good evidence or an FDA approval for treating depression too. And primary adverse effects are the worsening metabolic profile.
References:
- Toh, S., Li, Q., Cheetham, T. C., Cooper, W. O., Davis, R. L., Dublin, S., Hammad, T. A., Li, D., Pawloski, P. A., Pinheiro, S. P., Raebel, M. A., Scott, P. E., Smith, D. H., Bobo, W. V., Lawrence, J. M., Dashevsky, I., Haffenreffer, K., Avalos, L. A., & Andrade, S. E. (2013). Prevalence and trends in the use of antipsychotic medications during pregnancy in the U.S., 2001–2007: A population-based study of 585,615 deliveries. Archives of Women's Mental Health, 16(2), 149-157.
- Habermann, F., Fritzsche, J., Fuhlbrück, F., Wacker, E., Allignol, A., Weber-Schoendorfer, C., Meister, R., & Schaefer, C. (2013). Atypical antipsychotic drugs and pregnancy outcome. Journal of Clinical Psychopharmacology, 33(4), 453-462.
- Vigod, S. N., Gomes, T., Wilton, A. S., Taylor, V. H., & Ray, J. G. (2015). Antipsychotic drug use in pregnancy: High dimensional, propensity matched, population based cohort study. BMJ, 350(may11 5), h2298-h2298.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 3 of 8
So how do we apply these principles to use in pregnancy? Starting with the first-generation antipsychotics, we have a long history of clinical use in pregnancy. So in summary, with the first-generation antipsychotics, the pregnancy course, not finding an increased risk of miscarriage or stillbirth. Possibly an increased risk of preterm delivery. Again, it's unclear. These are used in women not just with bipolar disorder but also schizophrenia and there can be risks of behavior or the disease itself. Neonatal outcomes with first-generation antipsychotics include a small risk of transient abnormal muscle movements. So possible postnatal adaptation could be called for haloperidol and risperidone. They're mostly nervous system concerns, jitteriness or somnolence, occasionally seizures, in one study up to 10%. And these symptoms like we see with lithium, they seem to be associated with higher doses. So again, using that lowest effective dose as possible. Teratogenicity, we're not finding an increase above the population norm with the first-generation typical antipsychotics. And while long-term neurodevelopmental data are limited, what we do know is reassuring at this point. Haloperidol in particular has been a first-generation antipsychotic that has been used in pregnancy.
References:
- Coughlin, C. G., Blackwell, K. A., Bartley, C., Hay, M., Yonkers, K. A., & Bloch, M. H. (2015). Obstetric and neonatal outcomes after antipsychotic medication exposure in pregnancy. Obstetrics & Gynecology, 125(5), 1224-1235.
- Huybrechts, K. F., Hernández-Díaz, S., Patorno, E., Desai, R. J., Mogun, H., Dejene, S. Z., Cohen, J. M., Panchaud, A., Cohen, L., & Bateman, B. T. (2016). Antipsychotic use in pregnancy and the risk for congenital malformations. JAMA Psychiatry, 73(9), 938.
- Vigod, S. N., Gomes, T., Wilton, A. S., Taylor, V. H., & Ray, J. G. (2015). Antipsychotic drug use in pregnancy: High dimensional, propensity matched, population based cohort study. BMJ, 350(may11 5), h2298-h2298.
Slide 4 of 8
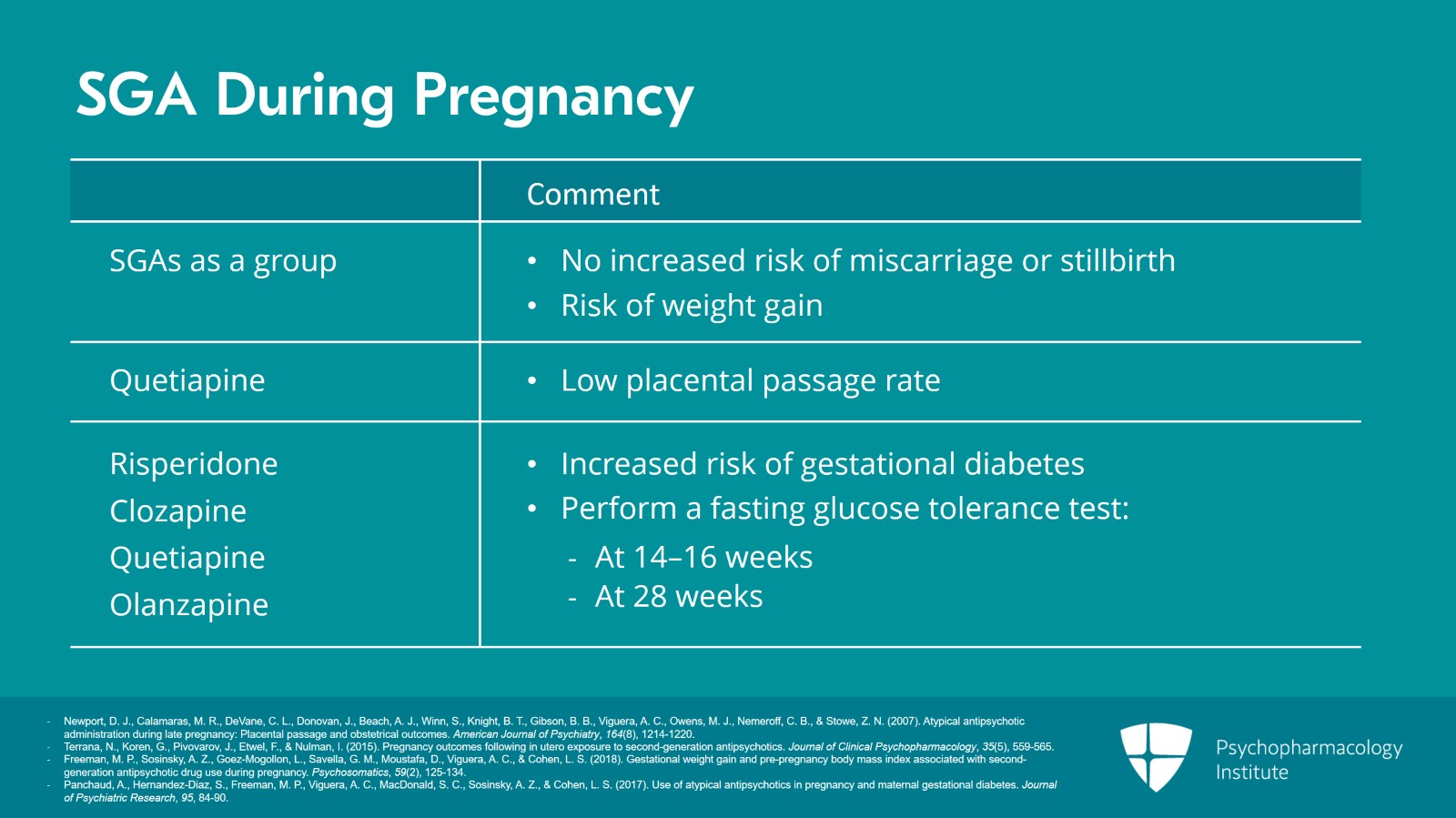
For the second-generation antipsychotics, these are more commonly prescribed these days and that includes in pregnancy. They're increasingly prescribed in pregnancy. Well, I'm going to talk about their data in general. There was a study that showed that quetiapine has been reported to have a relatively low placental passage rate compared to the others. Regardless of that, second-generation antipsychotics have increasing data and this is often reassuring on their use in pregnancy. So in the pregnancy course, again, we're not finding an increased risk of miscarriage or stillbirth. We do see that obesity or weight gain above the guidelines is a risk. But this isn't necessarily greater than when no atypical antipsychotic was used. Gestational diabetes specific for atypical antipsychotics is seen with risperidone, clozapine. And then again, higher doses, we're finding increased risk. So higher doses are associated with increased gestational diabetes risk for quetiapine and olanzapine. Because of the increased risk of gestational diabetes, it is recommended that women on atypical antipsychotics should have a fasting glucose tolerance test in the second trimester between 14 and 16 weeks and in the third trimester approximately 28 weeks. Just a little review on this, this is the more rigorous glucose testing. Standard testing in the second trimester is the glucose challenge I spoke of above but this glucose tolerance test which is generally in the purview of the OB is a more involved testing looking at glucose levels and metabolism in pregnancy. It's generally under the OB's purview and so again it behooves the psych prescriber to have release of information or communication with the OB. Some of these data are a bit complicated because women on atypicals often enter pregnancy with higher obesity and diabetes rates as well as other risk factors that women not on antipsychotics don't have. And as we see, these are risks in the metabolic profile that we know are in general risks for atypicals so it fits with one's reasoning on what the risks in pregnancy would be as well.
References:
- Newport, D. J., Calamaras, M. R., DeVane, C. L., Donovan, J., Beach, A. J., Winn, S., Knight, B. T., Gibson, B. B., Viguera, A. C., Owens, M. J., Nemeroff, C. B., & Stowe, Z. N. (2007). Atypical antipsychotic administration during late pregnancy: Placental passage and obstetrical outcomes. American Journal of Psychiatry, 164(8), 1214-1220.
- Terrana, N., Koren, G., Pivovarov, J., Etwel, F., & Nulman, I. (2015). Pregnancy outcomes following in utero exposure to second-generation antipsychotics. Journal of Clinical Psychopharmacology, 35(5), 559-565.
- Freeman, M. P., Sosinsky, A. Z., Goez-Mogollon, L., Savella, G. M., Moustafa, D., Viguera, A. C., & Cohen, L. S. (2018). Gestational weight gain and pre-pregnancy body mass index associated with second-generation antipsychotic drug use during pregnancy. Psychosomatics, 59(2), 125-134.
- Panchaud, A., Hernandez-Diaz, S., Freeman, M. P., Viguera, A. C., MacDonald, S. C., Sosinsky, A. Z., & Cohen, L. S. (2017). Use of atypical antipsychotics in pregnancy and maternal gestational diabetes. Journal of Psychiatric Research, 95, 84-90.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 5 of 8
For fetal or neonatal outcomes, the data are mixed. Perhaps small versus large versus normal for gestational age. We're getting some mixed reports. Potentially preterm. Again, there are a lot of baseline risk factors that can be hard to sort out. There are reports of transient abnormal muscle movements in about 10% to 15% of newborns. And this can include deficits of neuromotor performance, hypertonicity and stiffness, tremors. And these are compared to those exposed to antidepressants or non-psychotropic medications. Also found with atypicals can be jitteriness, somnolence or seizure like we saw with the first-generation antipsychotics. Moving on to teratogenicity, atypicals in whole have not been associated with increased teratogenicity. The exception is risperidone and potentially its metabolite, the paliperidone. While there are reports of potential increase of teratogenicity such as cardiac or oral cleft, it has been difficult to separate out other comorbid risk factors such as substance misuse or risk of polypharmacy that occur more often in women who are taking atypicals in pregnancy. So again, our data are limited by the limited rigor of our studies that we can design in pregnancy. Moving on to neurodevelopmental data for atypicals in pregnancy. At this point, the longest recorded is 12 months and it is reassuring.
References:
- Vigod, S. N., Gomes, T., Wilton, A. S., Taylor, V. H., & Ray, J. G. (2015). Antipsychotic drug use in pregnancy: High dimensional, propensity matched, population based cohort study. BMJ, 350(may11 5), h2298-h2298.
- Johnson, K. C., LaPrairie, J. L., Brennan, P. A., Stowe, Z. N., & Newport, D. J. (2012). Prenatal antipsychotic exposure and neuromotor performance during infancy. Archives of General Psychiatry, 69(8).
- Anderson, Kayla N., Elizabeth C. Ailes, Jennifer N. Lind, Cheryl S. Broussard, Rebecca H. Bitsko, Jan M. Friedman, William V. Bobo, Jennita Reefhuis, and Sarah C. Tinker. "Atypical antipsychotic use during pregnancy and birth defect risk: National Birth Defects Prevention Study, 1997–2011." Schizophrenia research 215 (2020): 81-88.
Slide 6 of 8
And our key points and conclusion. Again, there's no risk-free decision but the overall goal is to have the woman doing well. When possible, choose a medication and dose that has a known effectiveness for the patient.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 7 of 8
Discuss and document risks of untreated mood in pregnancy as well as the risks of medication use in pregnancy. When using antipsychotics, discuss with the woman the pregnancy course such as risks of excessive maternal weight gain, gestational diabetes as well as risks of teratogenicity particularly if used in that first trimester when the major organs are formed.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.