Slides and Transcript
Slide 2 of 30
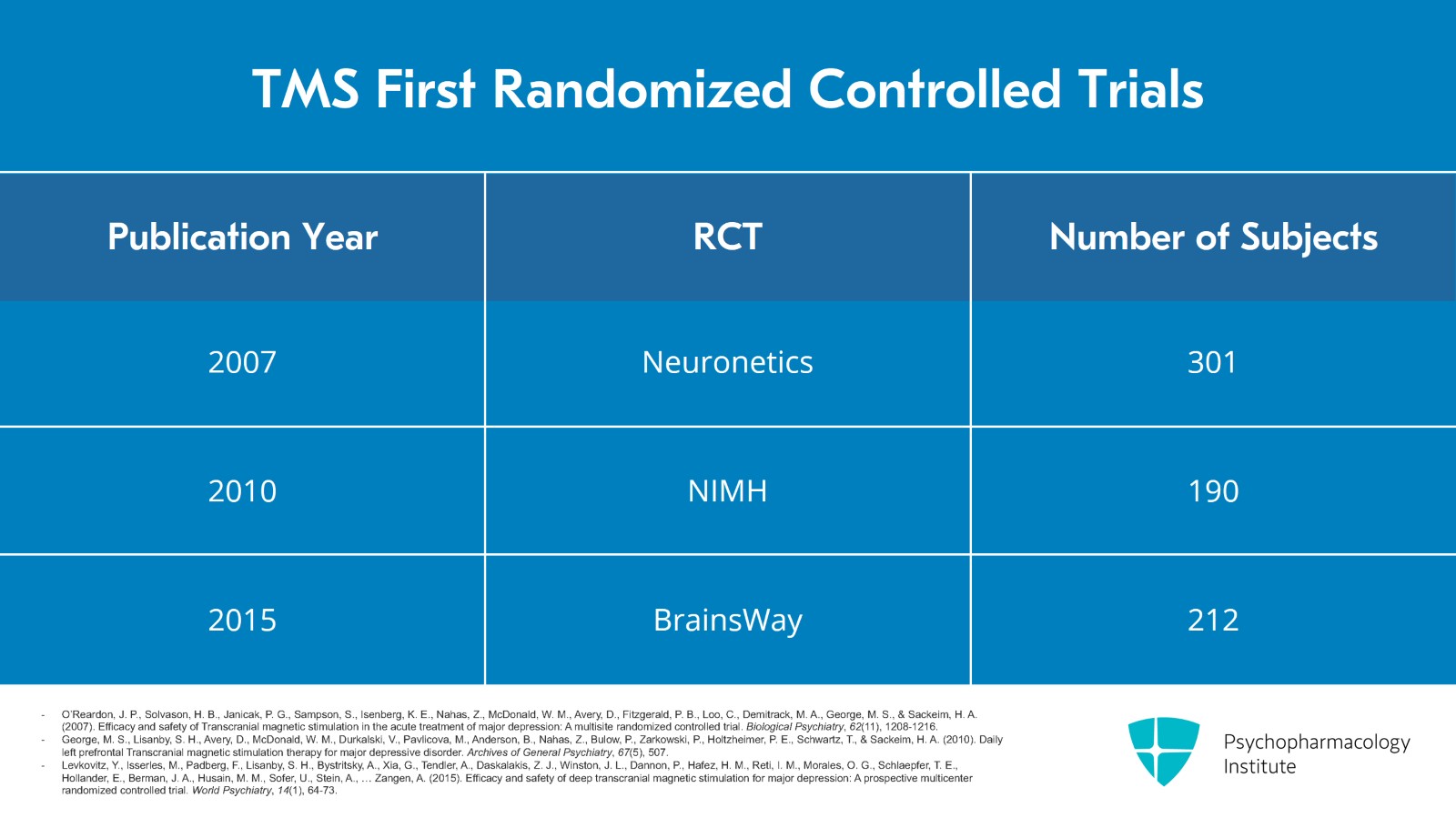
These are the first randomized controlled trials that were done. The original Neuronetics trial of 301 subjects was published in 2007 and that was the basis for the FDA approval. Mayo Clinic Rochester was one of the sites for this study. The National Institute of Mental Health funded a study of 190 subjects which was published in 2010. And in 2015, BrainsWay published their Deep TMS trial of 212 patients that was used for their device approval.
References:
- O’Reardon, J. P., Solvason, H. B., Janicak, P. G., Sampson, S., Isenberg, K. E., Nahas, Z., McDonald, W. M., Avery, D., Fitzgerald, P. B., Loo, C., Demitrack, M. A., George, M. S., & Sackeim, H. A. (2007). Efficacy and safety of Transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biological Psychiatry, 62(11), 1208-1216.
- George, M. S., Lisanby, S. H., Avery, D., McDonald, W. M., Durkalski, V., Pavlicova, M., Anderson, B., Nahas, Z., Bulow, P., Zarkowski, P., Holtzheimer, P. E., Schwartz, T., & Sackeim, H. A. (2010). Daily left prefrontal Transcranial magnetic stimulation therapy for major depressive disorder. Archives of General Psychiatry, 67(5), 507.
- Levkovitz, Y., Isserles, M., Padberg, F., Lisanby, S. H., Bystritsky, A., Xia, G., Tendler, A., Daskalakis, Z. J., Winston, J. L., Dannon, P., Hafez, H. M., Reti, I. M., Morales, O. G., Schlaepfer, T. E., Hollander, E., Berman, J. A., Husain, M. M., Sofer, U., Stein, A., … Zangen, A. (2015). Efficacy and safety of deep transcranial magnetic stimulation for major depression: A prospective multicenter randomized controlled trial. World Psychiatry, 14(1), 64-73.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 3 of 30
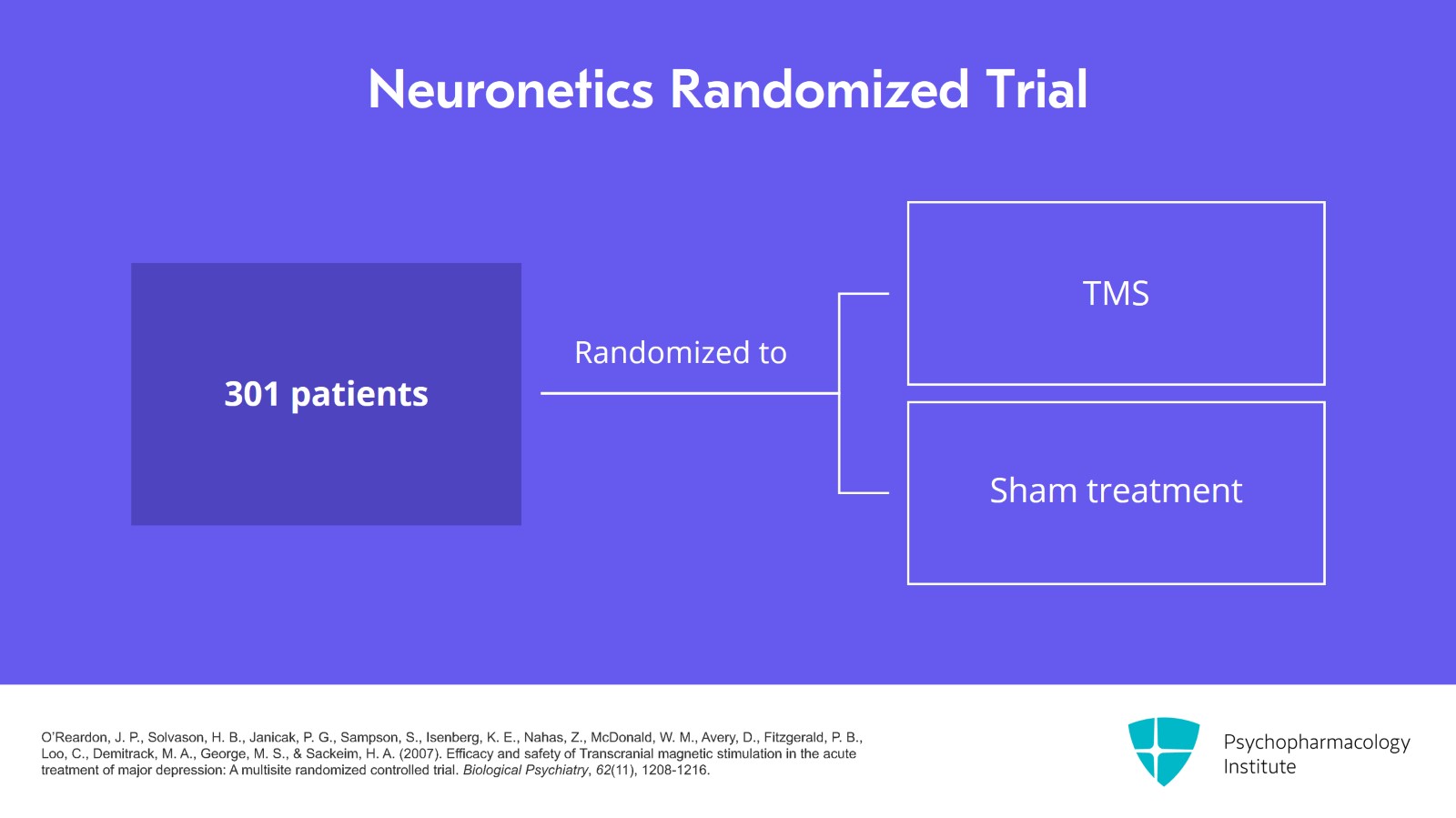
For the Neuronetics trial, they took 301 patients and randomized them to either TMS or a sham treatment.
References:
- O’Reardon, J. P., Solvason, H. B., Janicak, P. G., Sampson, S., Isenberg, K. E., Nahas, Z., McDonald, W. M., Avery, D., Fitzgerald, P. B., Loo, C., Demitrack, M. A., George, M. S., & Sackeim, H. A. (2007). Efficacy and safety of Transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biological Psychiatry, 62(11), 1208-1216.
Slide 4 of 30
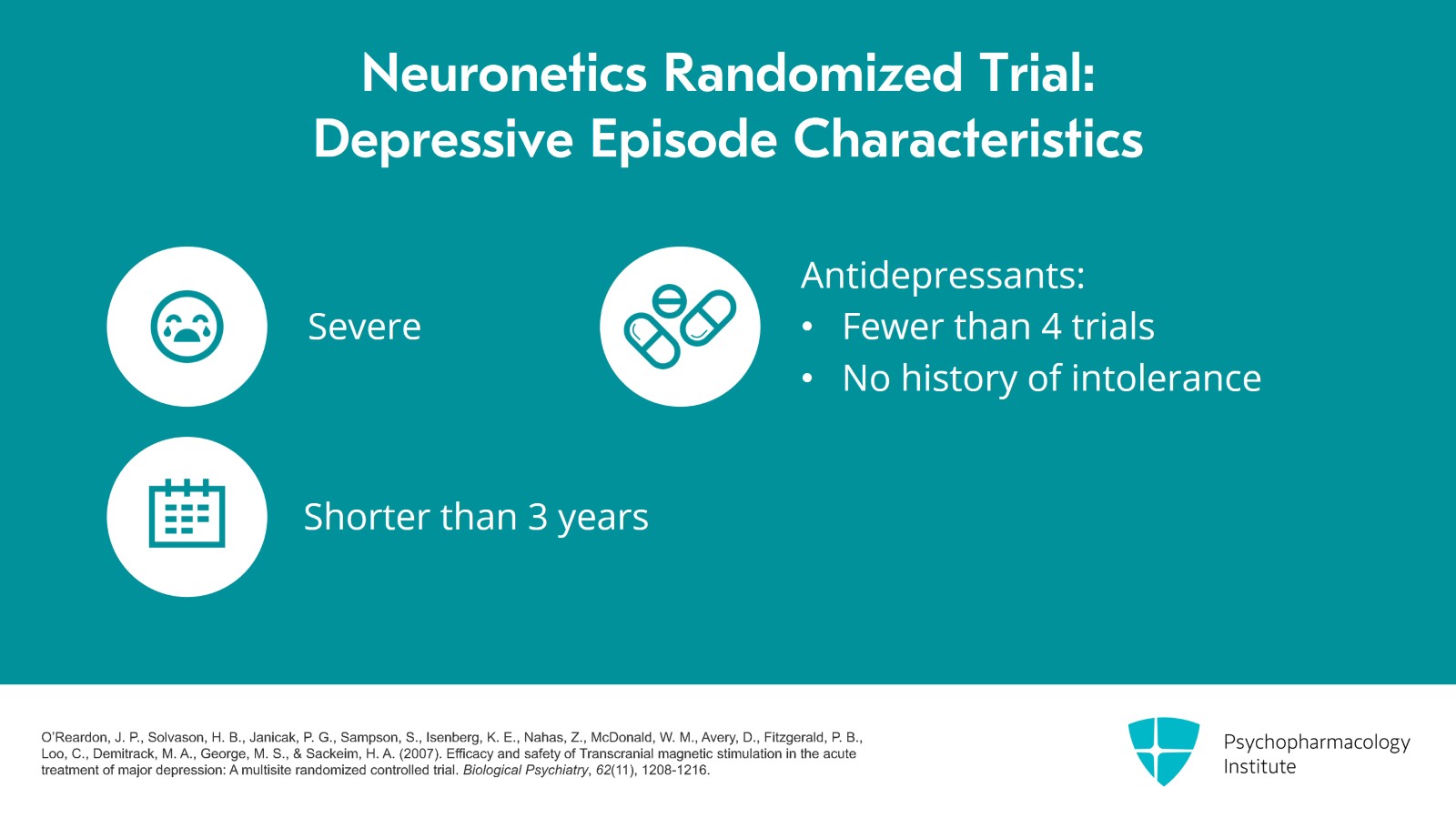
Patients had to have a certain severity of depression and their current episode of depression had to be less than three years and they couldn't have had more than four antidepressant trials or intolerance to antidepressants in that current episode of depression. So they didn't pick the people who are really, really treatment resistant with six or seven failed antidepressants.
References:
- O’Reardon, J. P., Solvason, H. B., Janicak, P. G., Sampson, S., Isenberg, K. E., Nahas, Z., McDonald, W. M., Avery, D., Fitzgerald, P. B., Loo, C., Demitrack, M. A., George, M. S., & Sackeim, H. A. (2007). Efficacy and safety of Transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biological Psychiatry, 62(11), 1208-1216.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 5 of 30
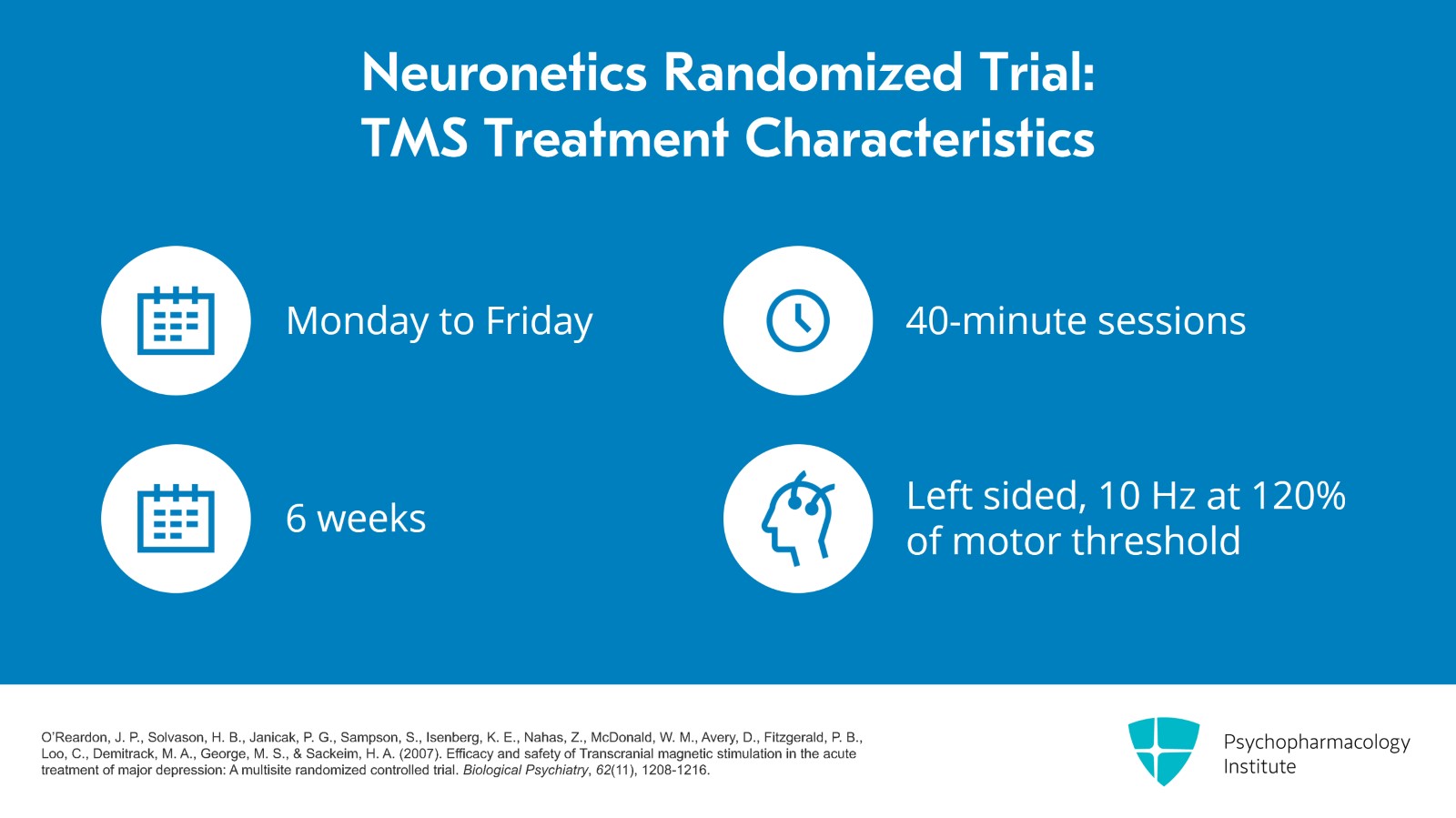
All of the patients were given Monday through Friday daily 40-minute sessions for six weeks and the treatment was left sided, 10 Hz at 120% of motor threshold.
References:
- O’Reardon, J. P., Solvason, H. B., Janicak, P. G., Sampson, S., Isenberg, K. E., Nahas, Z., McDonald, W. M., Avery, D., Fitzgerald, P. B., Loo, C., Demitrack, M. A., George, M. S., & Sackeim, H. A. (2007). Efficacy and safety of Transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biological Psychiatry, 62(11), 1208-1216.
Slide 6 of 30
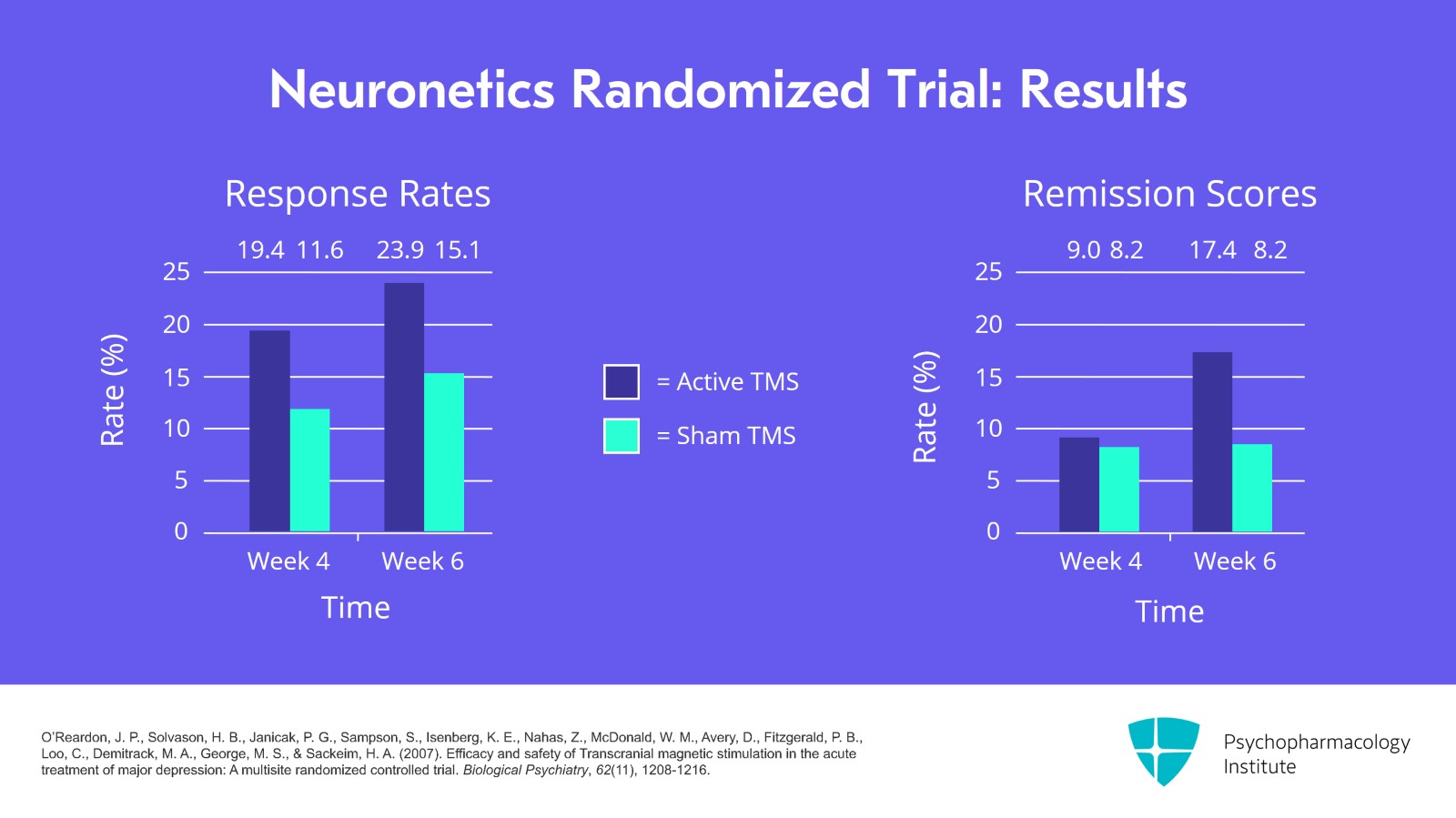
The results of the study are shown here. On the left side are the response rates. And if you look at week 4, you'll see that active versus sham starts to separate out. The response rate is 19.4% for active and 11.6% for sham. At week 6, it separates out even more, 23.9% for active versus 15.1% for sham. If you look at the right side at the remission scores, that doesn't separate out until week 6 where you have 17.4% of patients getting active treatment in remission compared to only 8.2% who received sham with remission.
References:
- O’Reardon, J. P., Solvason, H. B., Janicak, P. G., Sampson, S., Isenberg, K. E., Nahas, Z., McDonald, W. M., Avery, D., Fitzgerald, P. B., Loo, C., Demitrack, M. A., George, M. S., & Sackeim, H. A. (2007). Efficacy and safety of Transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biological Psychiatry, 62(11), 1208-1216.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 7 of 30
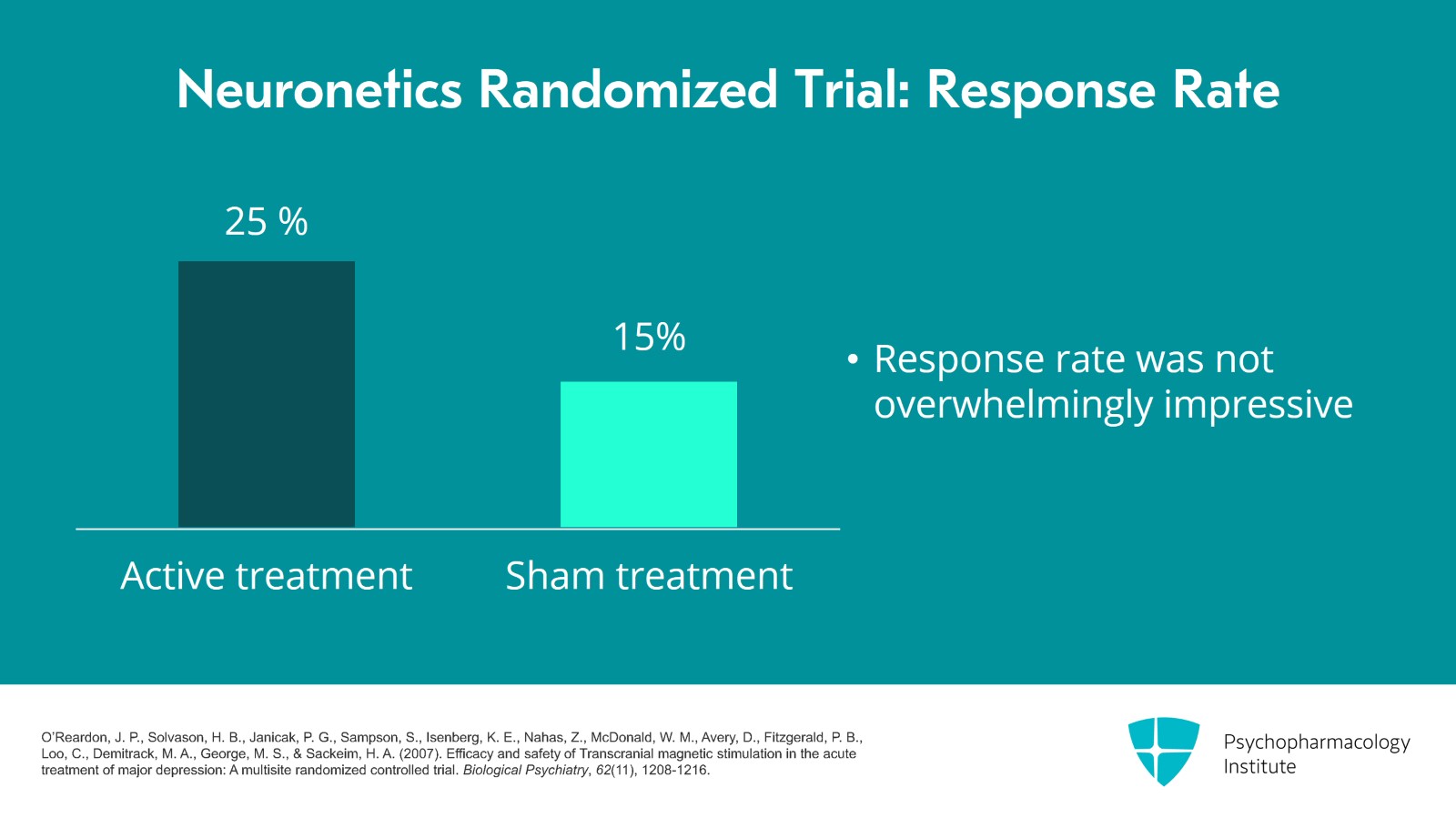
So let's pause and think about these numbers. Let's look at the response rate and round the numbers to 25% got better when they got the active treatment and 15% got better with sham. So what are some ways to think about these results? The overall response rate, is it impressive? If you have depression and you were offered a treatment which had a one in four chance of helping, would you be really excited? The numbers aren't overwhelmingly impressive but keep in mind that it's a randomized trial and we might expect lower rates for randomized trials versus open-label trials. Also, remember these are patients with treatment-resistant depression.
References:
- O’Reardon, J. P., Solvason, H. B., Janicak, P. G., Sampson, S., Isenberg, K. E., Nahas, Z., McDonald, W. M., Avery, D., Fitzgerald, P. B., Loo, C., Demitrack, M. A., George, M. S., & Sackeim, H. A. (2007). Efficacy and safety of Transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biological Psychiatry, 62(11), 1208-1216.
Slide 8 of 30
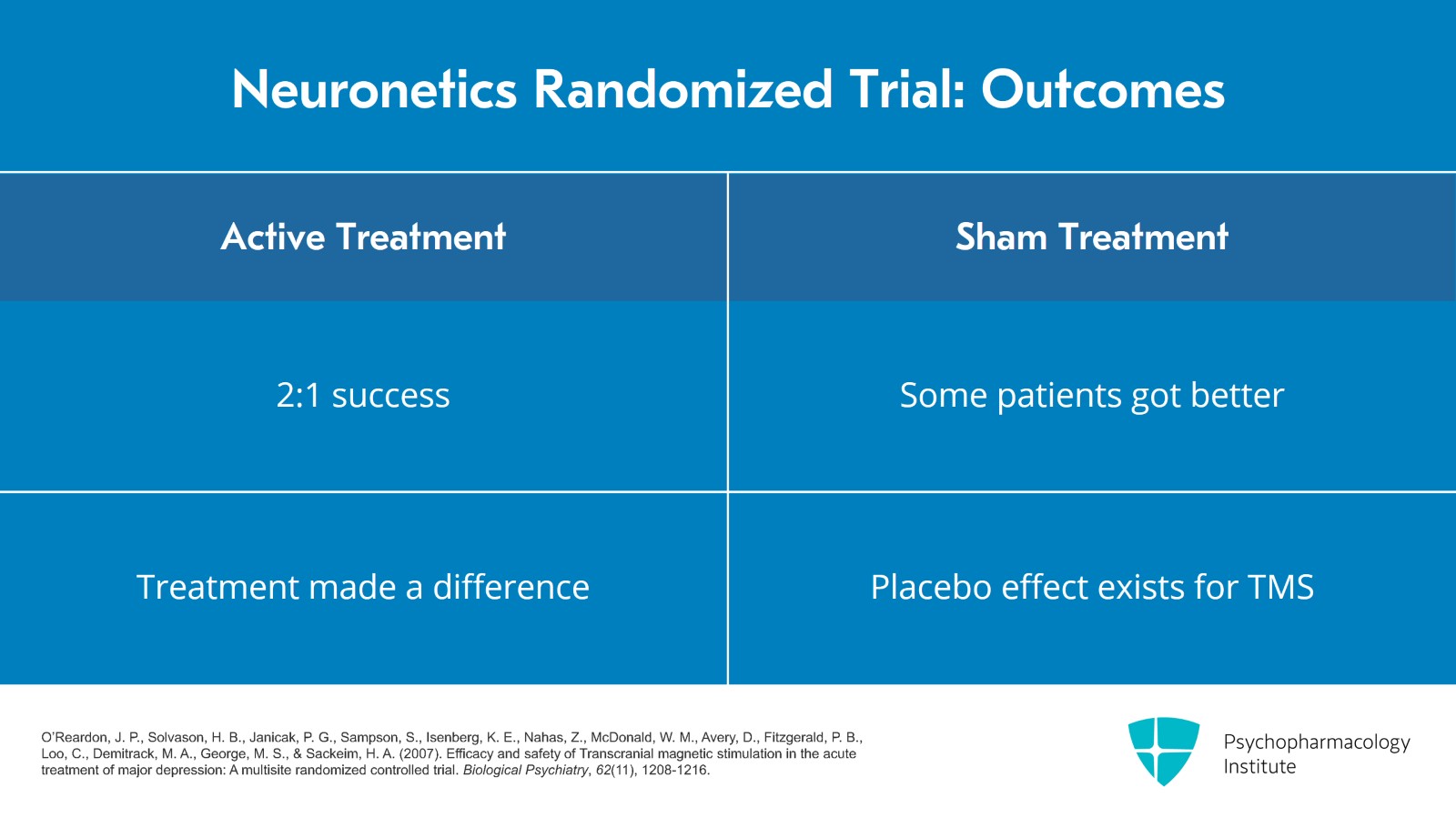
Then the next question, did the treatment work? Well, it was about two to one success for patients who received active versus sham. So the treatment arm certainly got better and treatment certainly made a difference. But a certain number of patients who got sham also got better which points out that the placebo effect exists even for TMS.
References:
- O’Reardon, J. P., Solvason, H. B., Janicak, P. G., Sampson, S., Isenberg, K. E., Nahas, Z., McDonald, W. M., Avery, D., Fitzgerald, P. B., Loo, C., Demitrack, M. A., George, M. S., & Sackeim, H. A. (2007). Efficacy and safety of Transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biological Psychiatry, 62(11), 1208-1216.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 9 of 30
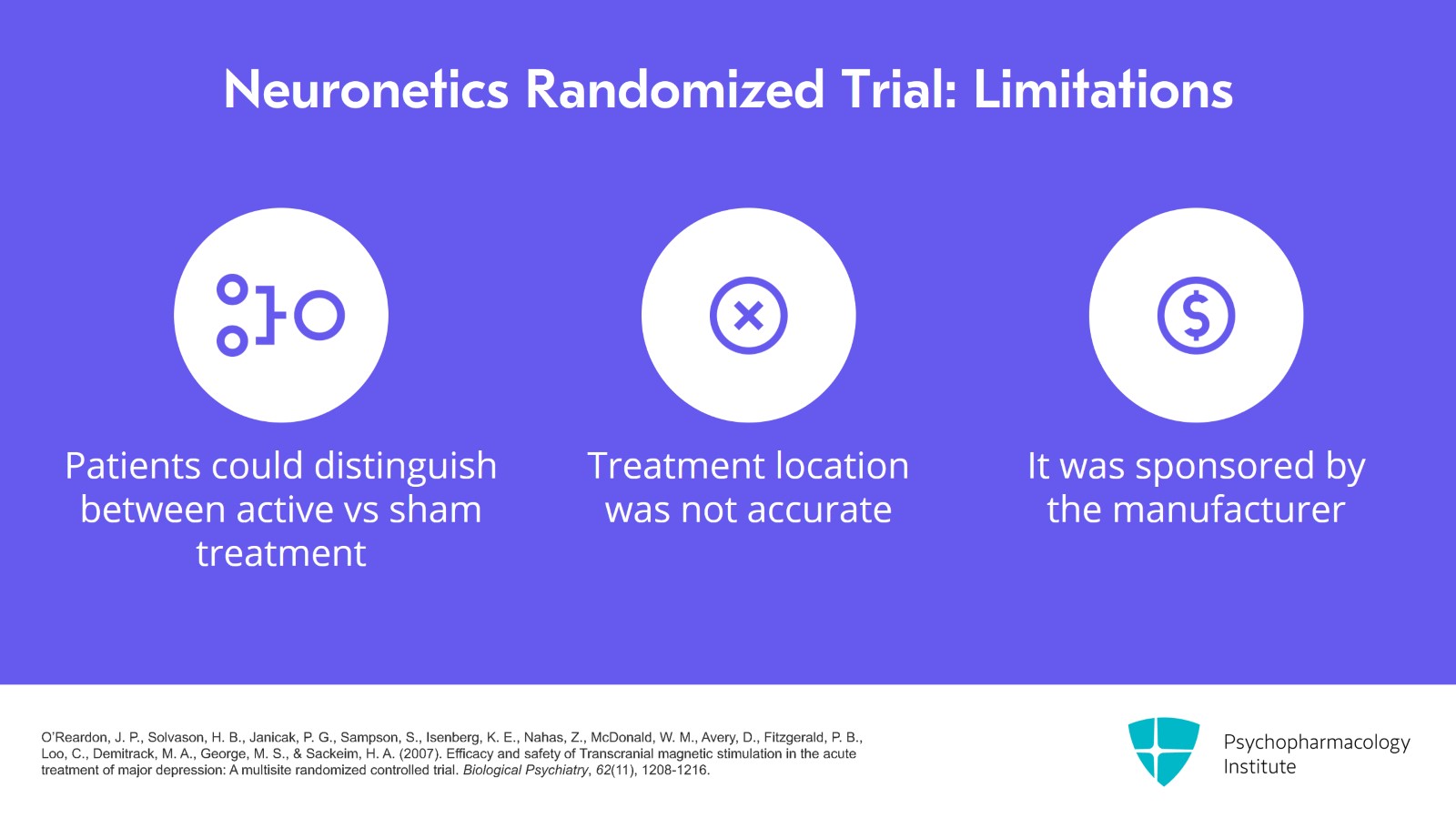
There were several weaknesses of the Neuronetics trial. One of them was that the patients could probably tell if they were getting the active versus sham treatment because the active would hurt a lot more than the sham. Another was that the treatment location of the dorsolateral prefrontal cortex was not accurate enough and then that it was sponsored by the company that made the device.
References:
- O’Reardon, J. P., Solvason, H. B., Janicak, P. G., Sampson, S., Isenberg, K. E., Nahas, Z., McDonald, W. M., Avery, D., Fitzgerald, P. B., Loo, C., Demitrack, M. A., George, M. S., & Sackeim, H. A. (2007). Efficacy and safety of Transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biological Psychiatry, 62(11), 1208-1216.
Slide 10 of 30
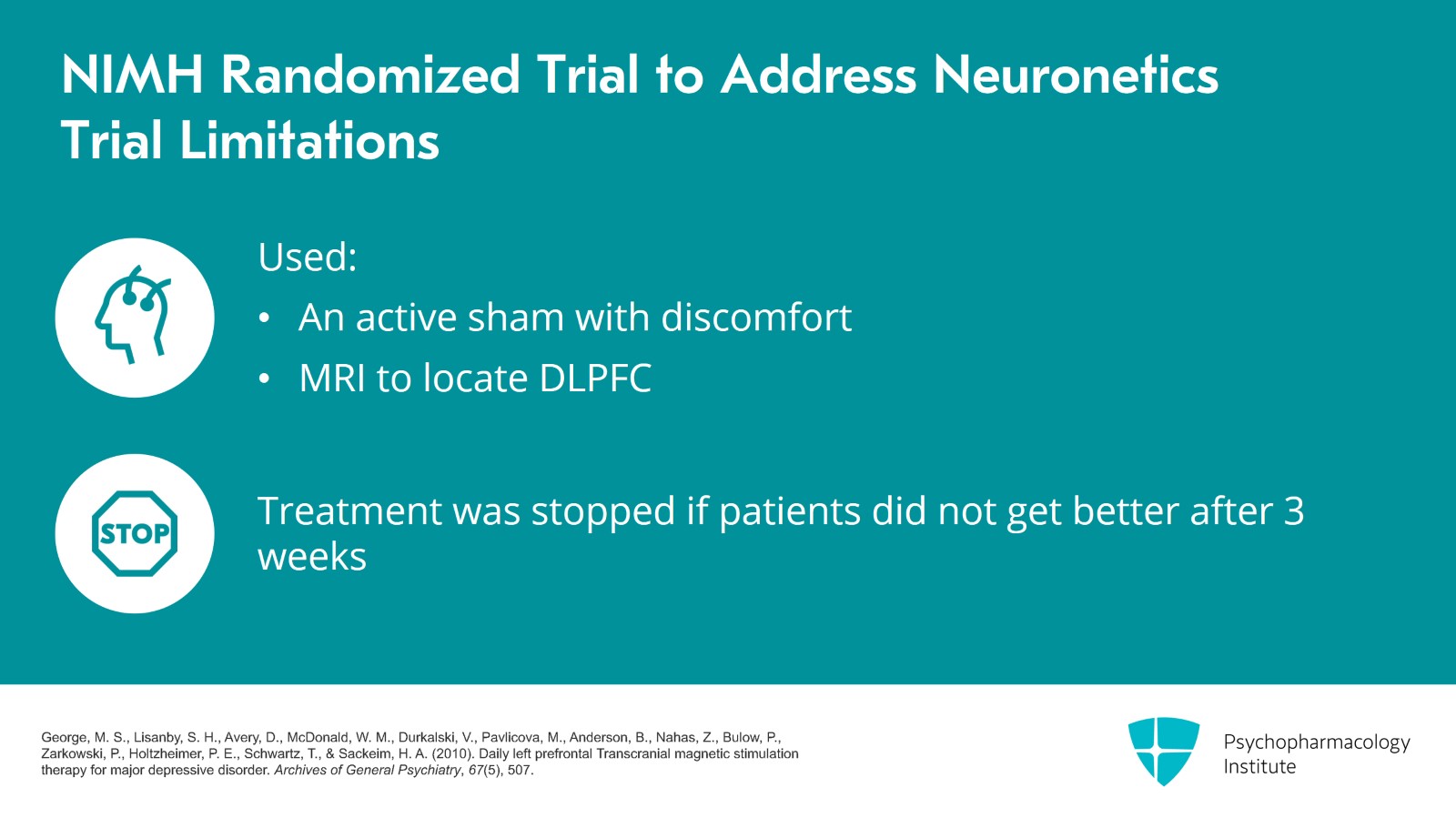
So the NIMH sponsored a randomized trial to address these concerns. They used an active sham where there is some discomfort with the sham and then they used MRI to locate the dorsolateral prefrontal cortex. The duration of the treatment was also different with patients stopping after three weeks if they did not get better and they had 190 patients in their analysis.
References:
- George, M. S., Lisanby, S. H., Avery, D., McDonald, W. M., Durkalski, V., Pavlicova, M., Anderson, B., Nahas, Z., Bulow, P., Zarkowski, P., Holtzheimer, P. E., Schwartz, T., & Sackeim, H. A. (2010). Daily left prefrontal Transcranial magnetic stimulation therapy for major depressive disorder. Archives of General Psychiatry, 67(5), 507.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 11 of 30
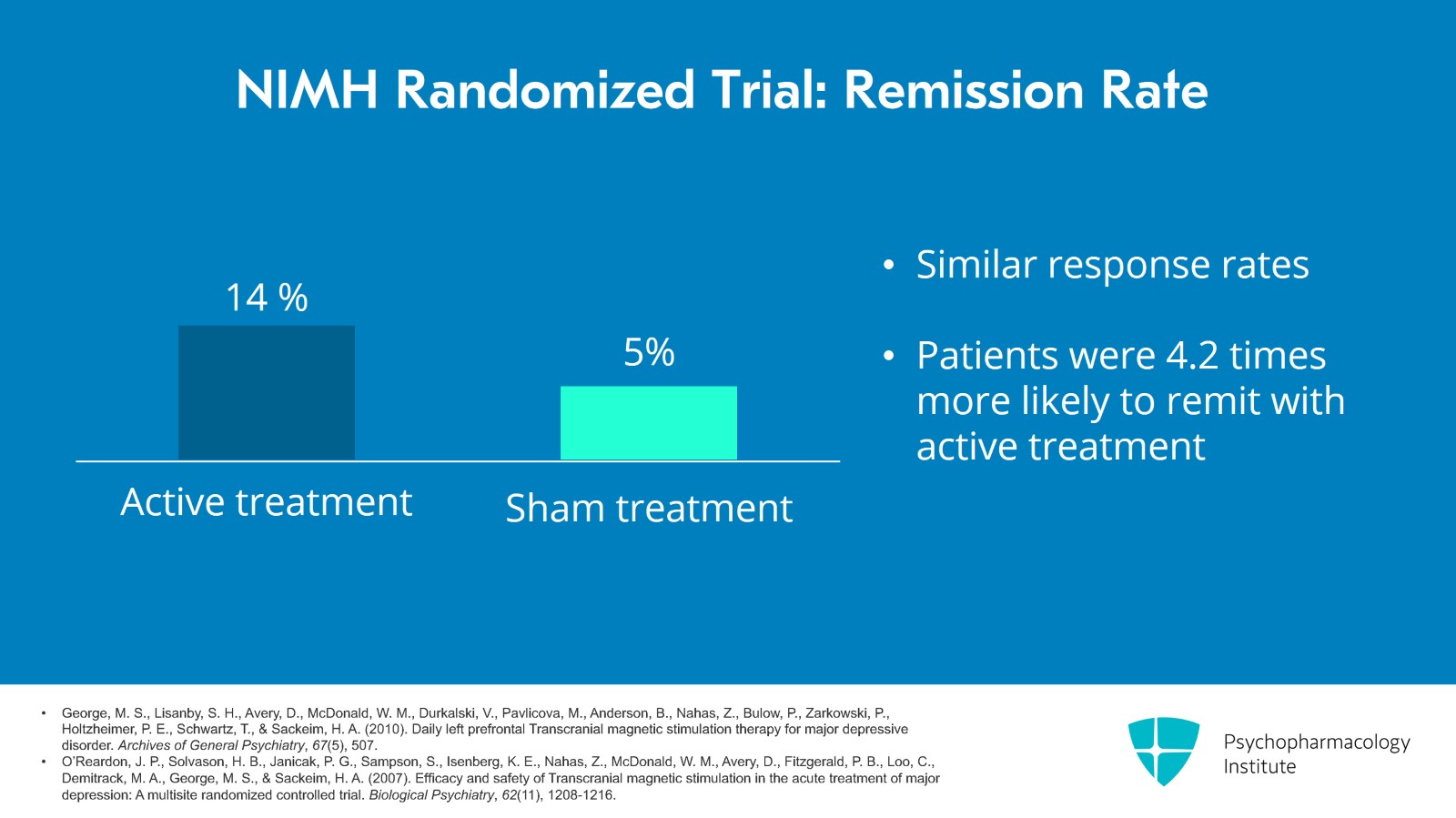
Here are the results of that study. The primary outcome measure was remission and 14% of patients who got active remitted versus 5% for sham. The response rates were similar. The odds ratio showed it was 4.2 times more likely to remit if you were getting active treatment.
References:
- George, M. S., Lisanby, S. H., Avery, D., McDonald, W. M., Durkalski, V., Pavlicova, M., Anderson, B., Nahas, Z., Bulow, P., Zarkowski, P., Holtzheimer, P. E., Schwartz, T., & Sackeim, H. A. (2010). Daily left prefrontal Transcranial magnetic stimulation therapy for major depressive disorder. Archives of General Psychiatry, 67(5), 507.
- O’Reardon, J. P., Solvason, H. B., Janicak, P. G., Sampson, S., Isenberg, K. E., Nahas, Z., McDonald, W. M., Avery, D., Fitzgerald, P. B., Loo, C., Demitrack, M. A., George, M. S., & Sackeim, H. A. (2007). Efficacy and safety of Transcranial magnetic stimulation in the acute treatment of major depression: A multisite randomized controlled trial. Biological Psychiatry, 62(11), 1208-1216.
Slide 12 of 30
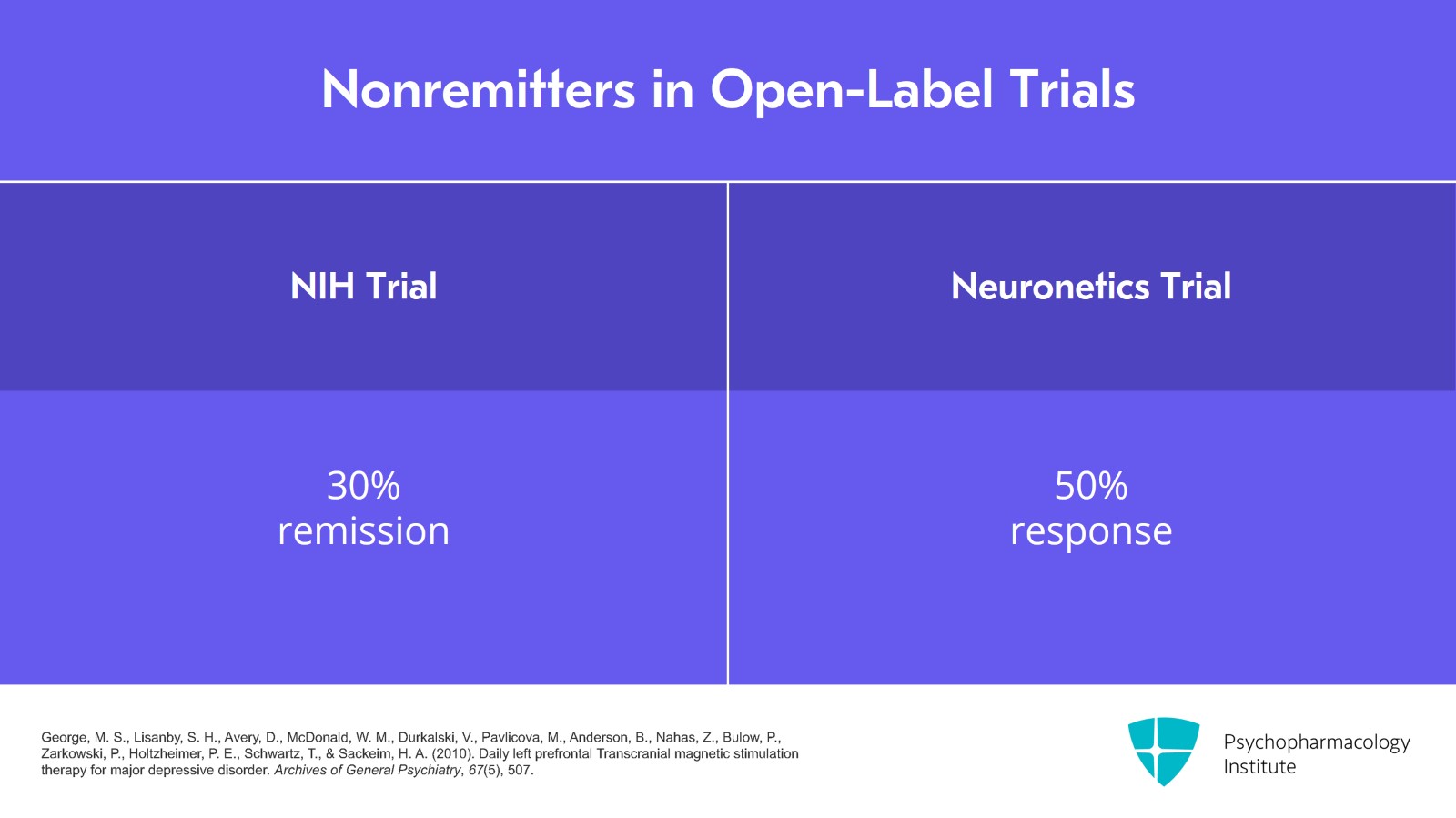
There's another part of the study where those who didn't remit with the randomized trial could participate in open-label follow-up. So about 30% of those who didn't get better with TMS in the randomized part then achieved remission with open-label treatment. I should mention that in the Neuronetics trial there was also a similar part where those who didn't respond to randomized could get open-label treatment and it was about 50% response.
References:
- George, M. S., Lisanby, S. H., Avery, D., McDonald, W. M., Durkalski, V., Pavlicova, M., Anderson, B., Nahas, Z., Bulow, P., Zarkowski, P., Holtzheimer, P. E., Schwartz, T., & Sackeim, H. A. (2010). Daily left prefrontal Transcranial magnetic stimulation therapy for major depressive disorder. Archives of General Psychiatry, 67(5), 507.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 13 of 30
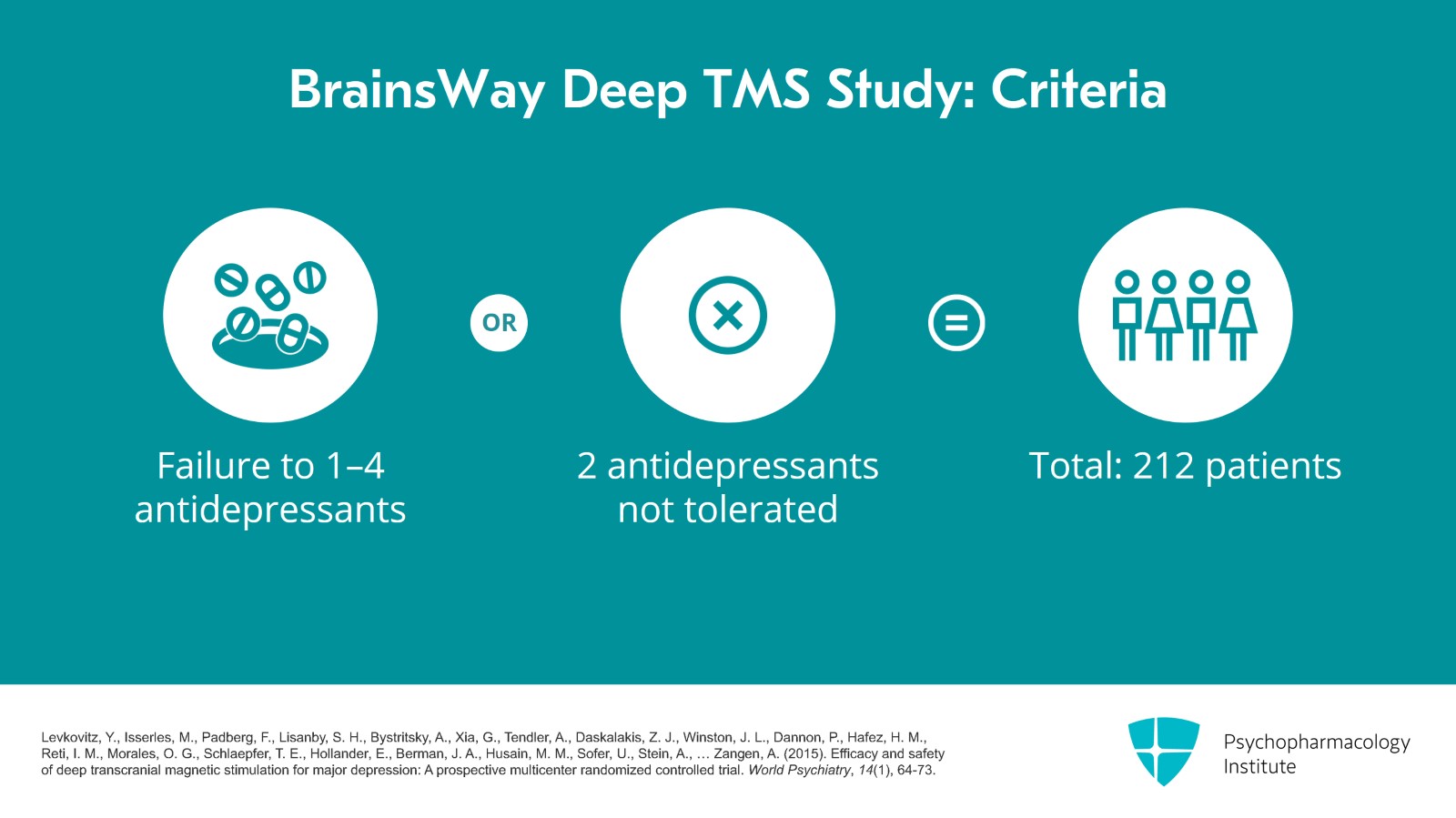
The next study is the BrainsWay Deep TMS study. Patients had to have failed one to four antidepressants or had at least two antidepressants that were not tolerated. They analyzed 212 patients.
References:
- Levkovitz, Y., Isserles, M., Padberg, F., Lisanby, S. H., Bystritsky, A., Xia, G., Tendler, A., Daskalakis, Z. J., Winston, J. L., Dannon, P., Hafez, H. M., Reti, I. M., Morales, O. G., Schlaepfer, T. E., Hollander, E., Berman, J. A., Husain, M. M., Sofer, U., Stein, A., … Zangen, A. (2015). Efficacy and safety of deep transcranial magnetic stimulation for major depression: A prospective multicenter randomized controlled trial. World Psychiatry, 14(1), 64-73.
Slide 14 of 30
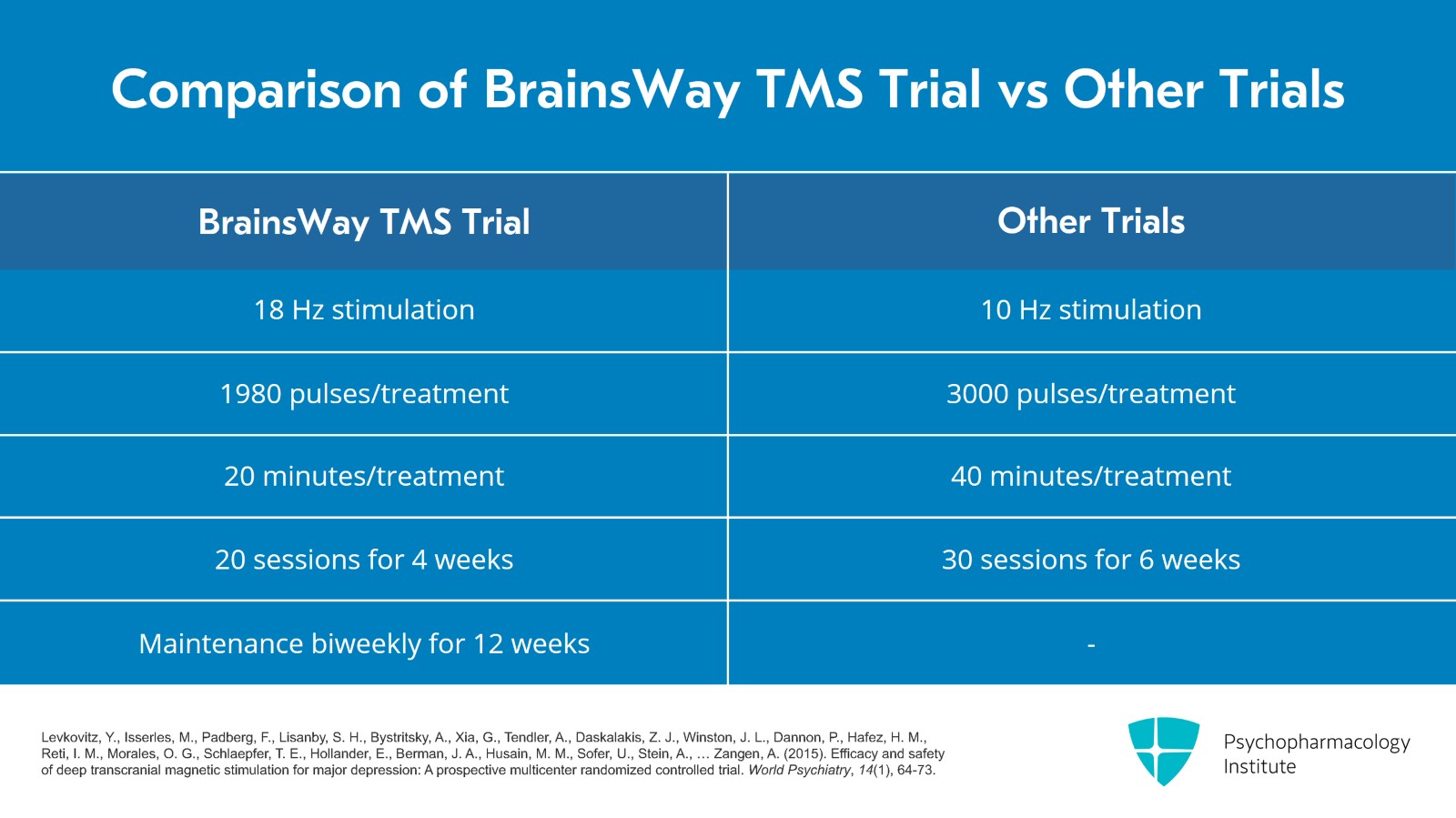
They used 18 Hz stimulation, 1980 pulses per treatment which took about 20 minutes for each treatment and 20 treatment sessions over four weeks. So these parameters are faster than the 10 Hz, 3000 pulse and 40-minute treatments that the other studies used and it's also shorter duration than the 30 treatments and six weeks of the other studies. There's a maintenance aspect to this research study also where they gave additional bi-weekly treatments for 12 weeks.
References:
- Levkovitz, Y., Isserles, M., Padberg, F., Lisanby, S. H., Bystritsky, A., Xia, G., Tendler, A., Daskalakis, Z. J., Winston, J. L., Dannon, P., Hafez, H. M., Reti, I. M., Morales, O. G., Schlaepfer, T. E., Hollander, E., Berman, J. A., Husain, M. M., Sofer, U., Stein, A., … Zangen, A. (2015). Efficacy and safety of deep transcranial magnetic stimulation for major depression: A prospective multicenter randomized controlled trial. World Psychiatry, 14(1), 64-73.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 15 of 30
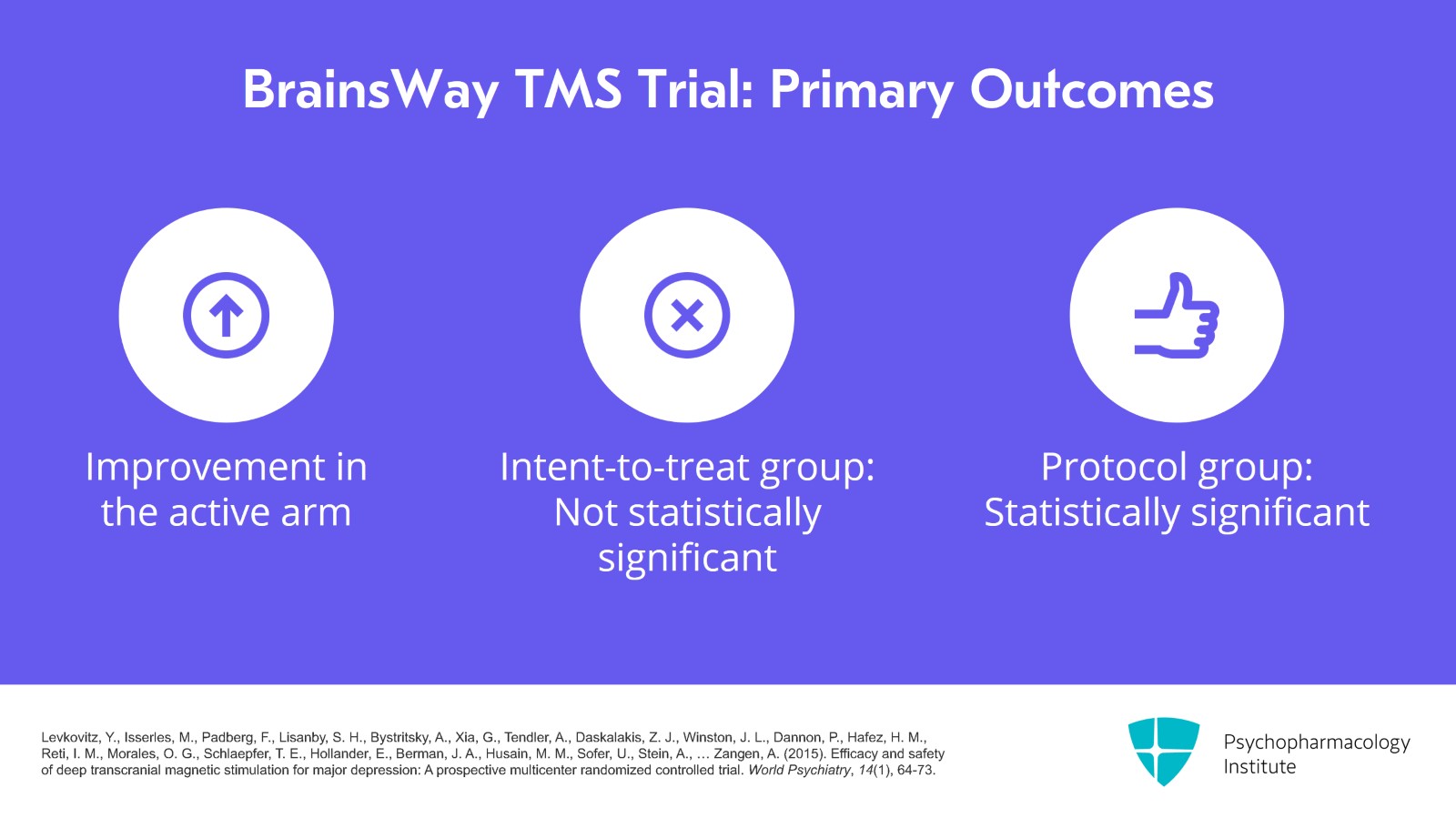
Here are the results of this study. The primary outcome was the change in the Hamilton Depression Rating Score in the acute phase and the improvement was only 3.1 points in the active arm and that just missed statistical significance in the intent-to-treat group, although in the protocol group it was statistically significant.
References:
- Levkovitz, Y., Isserles, M., Padberg, F., Lisanby, S. H., Bystritsky, A., Xia, G., Tendler, A., Daskalakis, Z. J., Winston, J. L., Dannon, P., Hafez, H. M., Reti, I. M., Morales, O. G., Schlaepfer, T. E., Hollander, E., Berman, J. A., Husain, M. M., Sofer, U., Stein, A., … Zangen, A. (2015). Efficacy and safety of deep transcranial magnetic stimulation for major depression: A prospective multicenter randomized controlled trial. World Psychiatry, 14(1), 64-73.
Slide 16 of 30
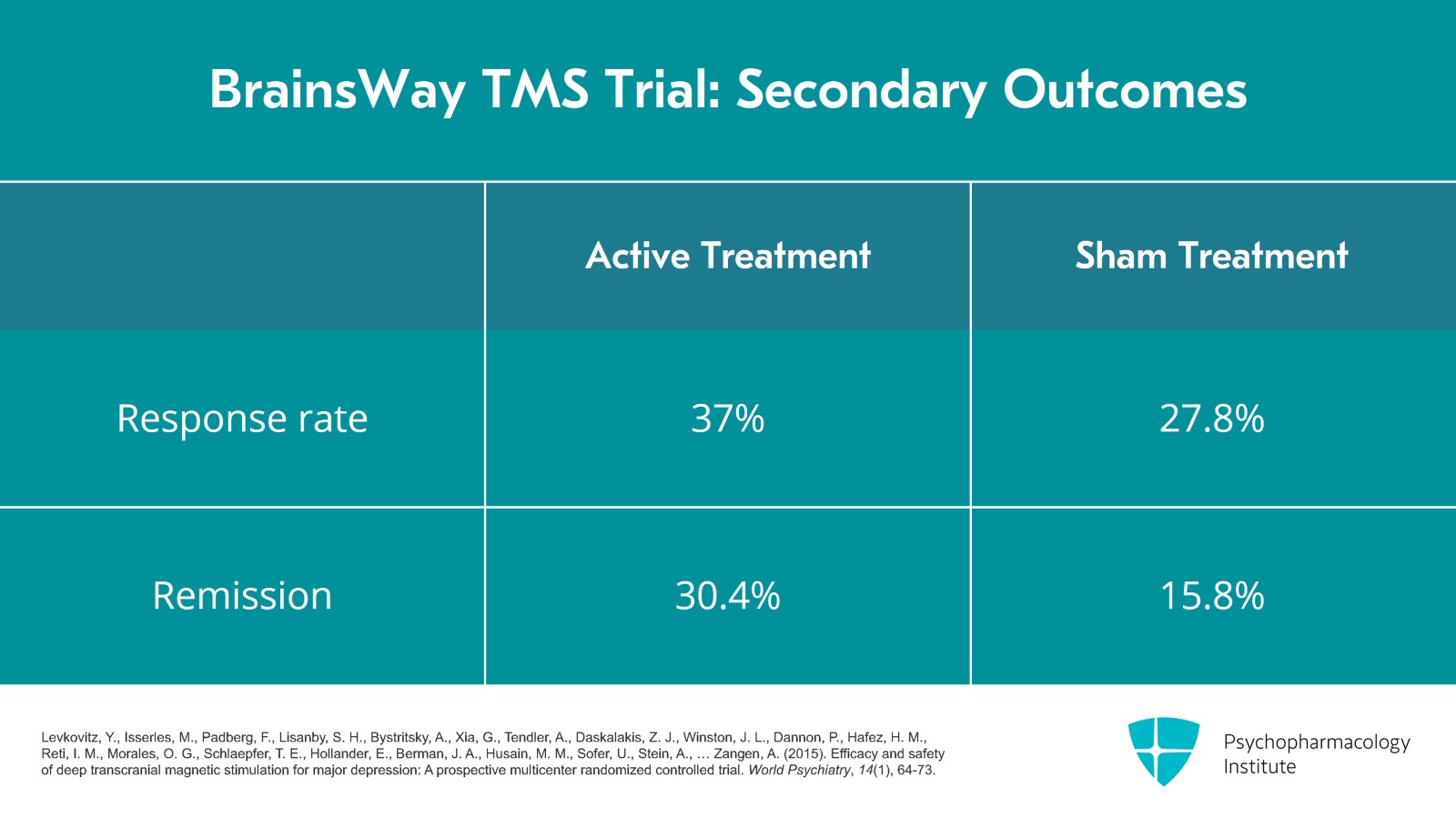
For the secondary outcomes, the response rate for active was 37% versus 27.8% for sham and then for remission, 30.4% versus 15.8%.
References:
- Levkovitz, Y., Isserles, M., Padberg, F., Lisanby, S. H., Bystritsky, A., Xia, G., Tendler, A., Daskalakis, Z. J., Winston, J. L., Dannon, P., Hafez, H. M., Reti, I. M., Morales, O. G., Schlaepfer, T. E., Hollander, E., Berman, J. A., Husain, M. M., Sofer, U., Stein, A., … Zangen, A. (2015). Efficacy and safety of deep transcranial magnetic stimulation for major depression: A prospective multicenter randomized controlled trial. World Psychiatry, 14(1), 64-73.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 17 of 30
Since FDA approval of TMS for depression, there are many reports of how patients do when they're directly given TMS. These are open-label or naturalistic studies. The first large one was published in 2012. The authors evaluated 307 patients at 42 different sites getting NeuroStar TMS. This study used the PHQ-9 to measure results and the response rate was 56.4% and the remission rate was 28.7%. This is a very practical number to use when I'm talking to patients about the success rate of TMS.
References:
- Carpenter, L. L., Janicak, P. G., Aaronson, S. T., Boyadjis, T., Brock, D. G., Cook, I. A., Dunner, D. L., Lanocha, K., Solvason, H. B., & Demitrack, M. A. (2012). Transcranial magnetic stimulation (Tms) for major depression: A multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depression and Anxiety, 29(7), 587-596.
Slide 18 of 30
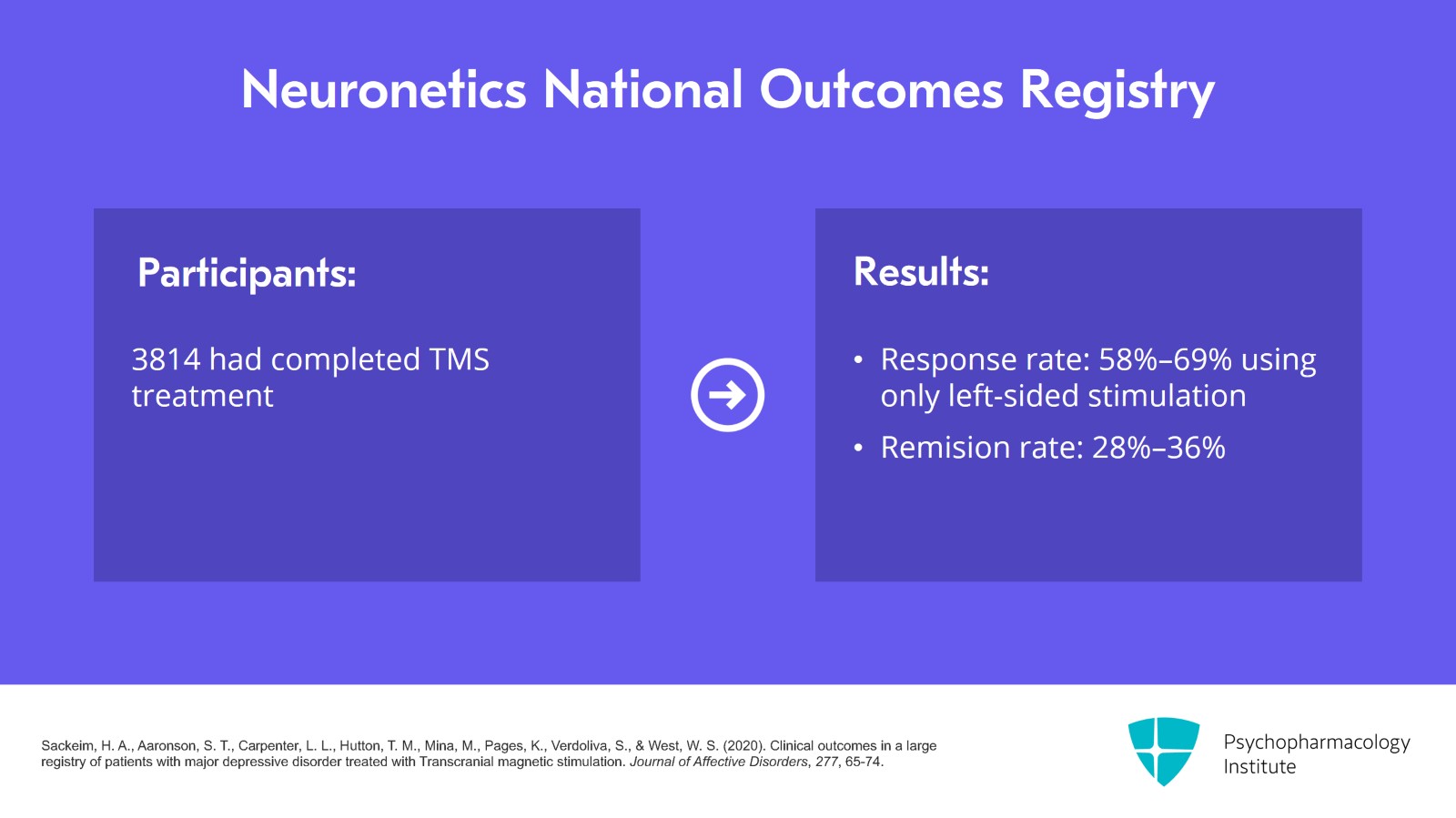
Neuronetics has a large National Outcomes Registry which keeps growing. In 2020, they published a report of 5010 patients with 3814 who completed TMS with at least 20 sessions and had a PHQ-9 score at the beginning and end of treatment. The response rate using PHQ-9 was between 58% to 69% with the highest response for completers using only left-sided stimulations as opposed to other stimulation protocols such as left- and right-sided stimulations. Remission rate was 28% to 36%. Their study did not find any difference in the response between younger and older patients.
References:
- Sackeim, H. A., Aaronson, S. T., Carpenter, L. L., Hutton, T. M., Mina, M., Pages, K., Verdoliva, S., & West, W. S. (2020). Clinical outcomes in a large registry of patients with major depressive disorder treated with Transcranial magnetic stimulation. Journal of Affective Disorders, 277, 65-74.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 19 of 30
With this registry, they also studied two different treatment protocols. The standard TMS protocol is daily 10 Hz stimulation over the left dorsolateral prefrontal cortex. Another treatment protocol is to alternate 10 Hz left dorsolateral prefrontal cortex with 1 Hz right dorsolateral prefrontal cortex, based on some studies showing that right dorsolateral prefrontal cortex can help with depression but also with anxiety. So this study compared patients in their National Registry which had one or the other protocol. There were six times as many patients who were treated with left unilateral compared to the bilateral protocol. The left unilateral protocol is what was used for FDA approval.
References:
- Aaronson, S. T., Carpenter, L. L., Hutton, T. M., Kraus, S., Mina, M., Pages, K., Shi, L., West, W. S., & Sackeim, H. A. (2022). Comparison of clinical outcomes with left unilateral and sequential bilateral Transcranial magnetic stimulation (TMS) treatment of major depressive disorder in a large patient registry. Brain Stimulation, 15(2), 326-336.
Slide 20 of 30
So after looking at the data in many different ways, their conclusion was that the left unilateral group had better outcomes than the bilateral group.
References:
- Aaronson, S. T., Carpenter, L. L., Hutton, T. M., Kraus, S., Mina, M., Pages, K., Shi, L., West, W. S., & Sackeim, H. A. (2022). Comparison of clinical outcomes with left unilateral and sequential bilateral Transcranial magnetic stimulation (TMS) treatment of major depressive disorder in a large patient registry. Brain Stimulation, 15(2), 326-336.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 21 of 30
In early 2023, they reported outcomes of anxious depression defined as major depressive disorder with a GAD-7 anxiety score of 10 or greater. The finding was that both anxiety and depression improved in patients who had high baseline anxiety. The improvement in depression wasn't as much as those who had low baseline anxiety but the improvement was still high, about 58% in the intent-to-treat group and 67% in the completer group. Keep in mind that approval was not for TMS for primary anxiety disorders as there's still not enough evidence for that condition.
References:
- Hutton, T. M., Aaronson, S. T., Carpenter, L. L., Pages, K., West, W. S., Kraemer, C., & Sackeim, H. A. (2023). The anxiolytic and antidepressant effects of Transcranial magnetic stimulation in patients with anxious depression. The Journal of Clinical Psychiatry, 84(1).
Slide 22 of 30
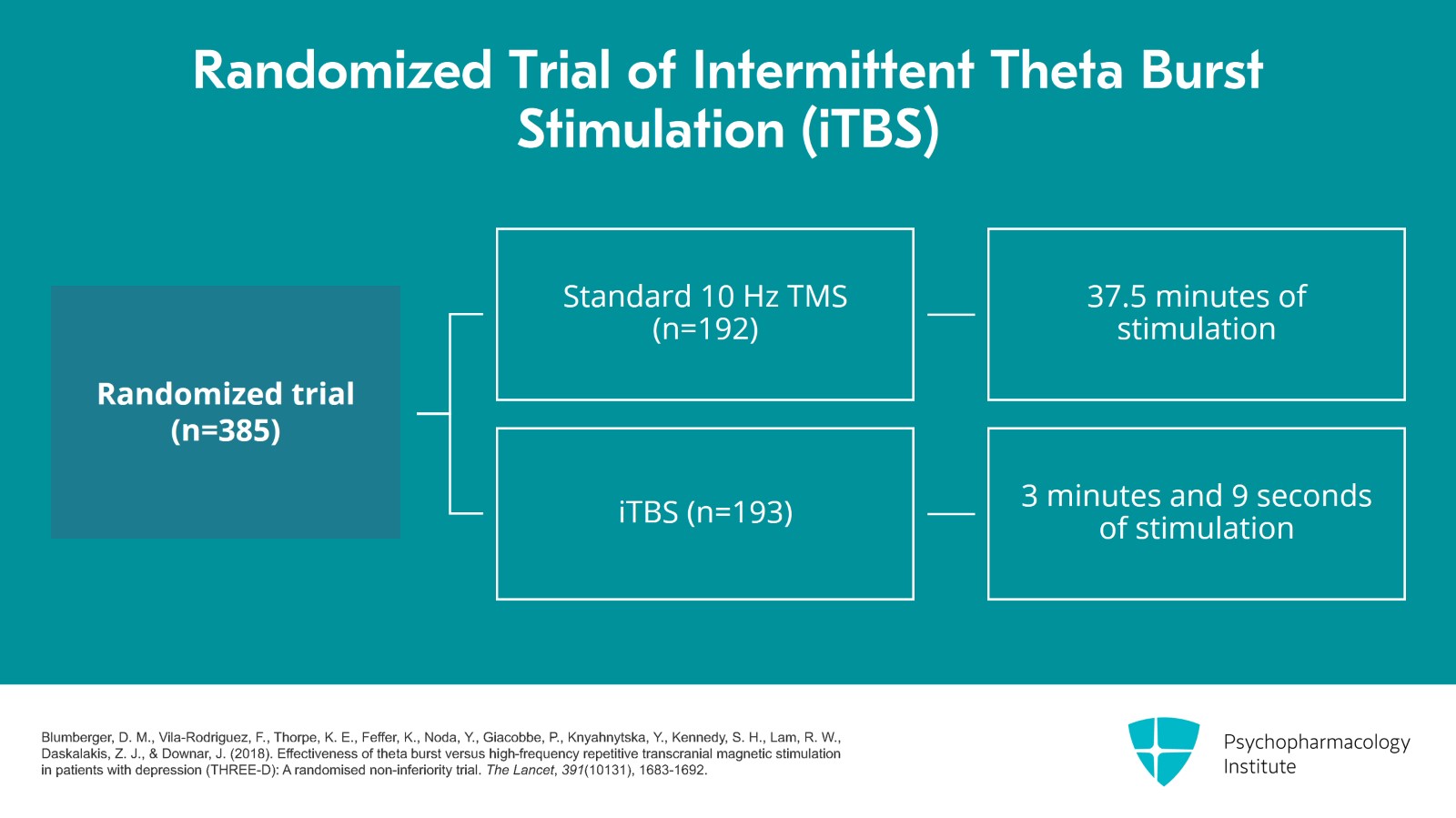
Now, let's look at the data which got iTBS approved. It was a randomized, non-inferiority trial of 385 adult patients ages 18 to 65 years and 192 were assigned to standard 10 Hz TMS and 193 assigned to iTBS. Each iTBS treatment was 3 minutes and 9 seconds versus 37.5 minutes for 10 Hz rTMS. Both groups received four to six weeks of five days per week treatment. If at four weeks, there was at least 30% improvement but not remission, the treatments continued to six weeks.
References:
- Blumberger, D. M., Vila-Rodriguez, F., Thorpe, K. E., Feffer, K., Noda, Y., Giacobbe, P., Knyahnytska, Y., Kennedy, S. H., Lam, R. W., Daskalakis, Z. J., & Downar, J. (2018). Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. The Lancet, 391(10131), 1683-1692.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 23 of 30
The primary outcome was the 17-item Hamilton Depression Rating Scale. With a non-inferiority margin of 2.25 points, the Hamilton Rating Scale baseline and end scores were basically the same for both groups as was the response rate.
References:
- Blumberger, D. M., Vila-Rodriguez, F., Thorpe, K. E., Feffer, K., Noda, Y., Giacobbe, P., Knyahnytska, Y., Kennedy, S. H., Lam, R. W., Daskalakis, Z. J., & Downar, J. (2018). Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. The Lancet, 391(10131), 1683-1692.
Slide 24 of 30
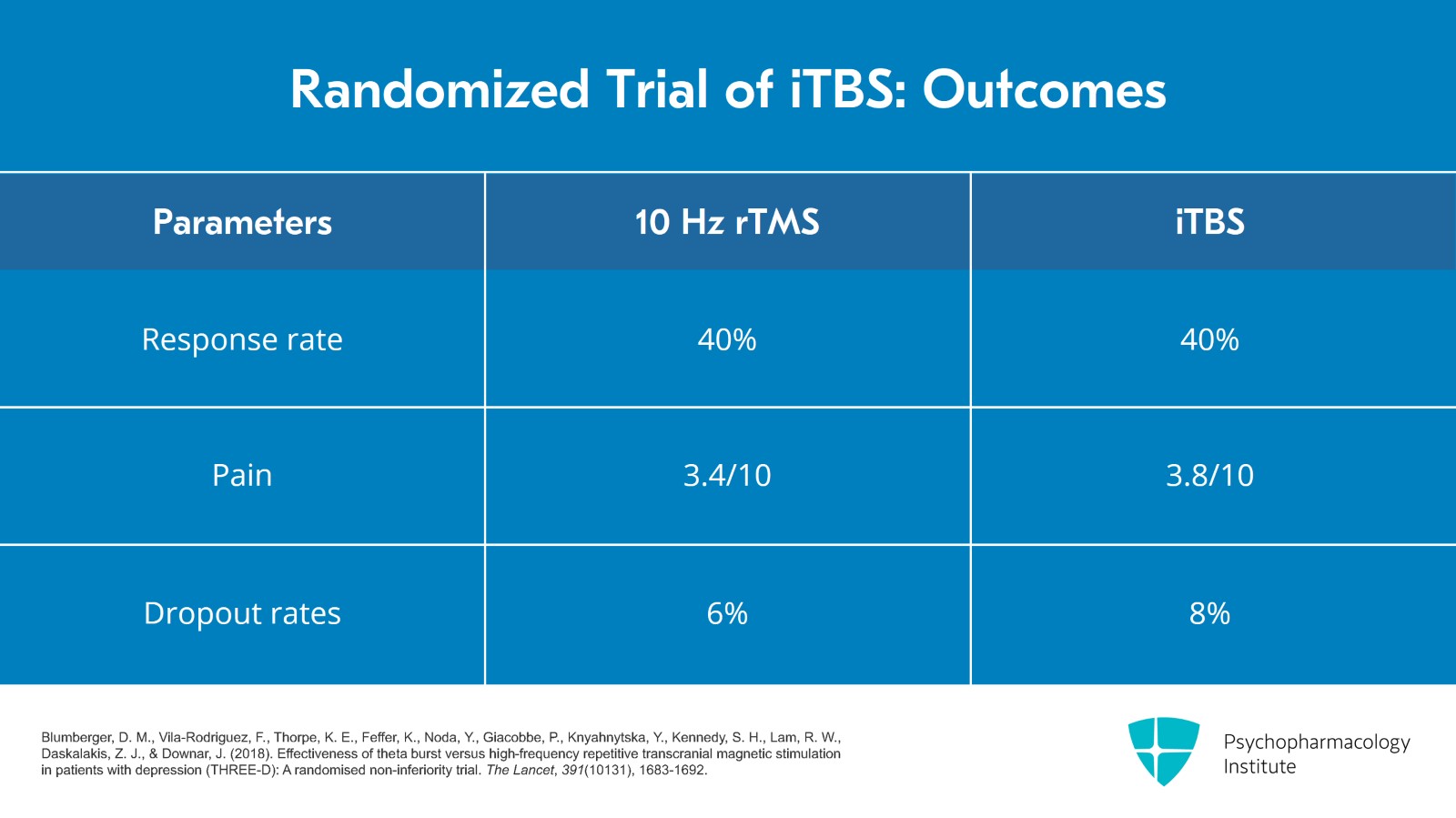
In this table, I included the response rates for the QIDS Self-Rating Scale that was also used and the response rate was 40% for both groups. Pain was rated higher in the TBS group. It was 3.8 out of 10 versus 3.4 out of 10 for the rTMS group and the dropout rates were similar with 8% in the iTBS and 6% in the TMS group.
References:
- Blumberger, D. M., Vila-Rodriguez, F., Thorpe, K. E., Feffer, K., Noda, Y., Giacobbe, P., Knyahnytska, Y., Kennedy, S. H., Lam, R. W., Daskalakis, Z. J., & Downar, J. (2018). Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. The Lancet, 391(10131), 1683-1692.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 25 of 30
So this is an important study which tells us that standard 10 Hz rTMS and iTBS have the same outcomes.
References:
- Blumberger, D. M., Vila-Rodriguez, F., Thorpe, K. E., Feffer, K., Noda, Y., Giacobbe, P., Knyahnytska, Y., Kennedy, S. H., Lam, R. W., Daskalakis, Z. J., & Downar, J. (2018). Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. The Lancet, 391(10131), 1683-1692.
Slide 26 of 30
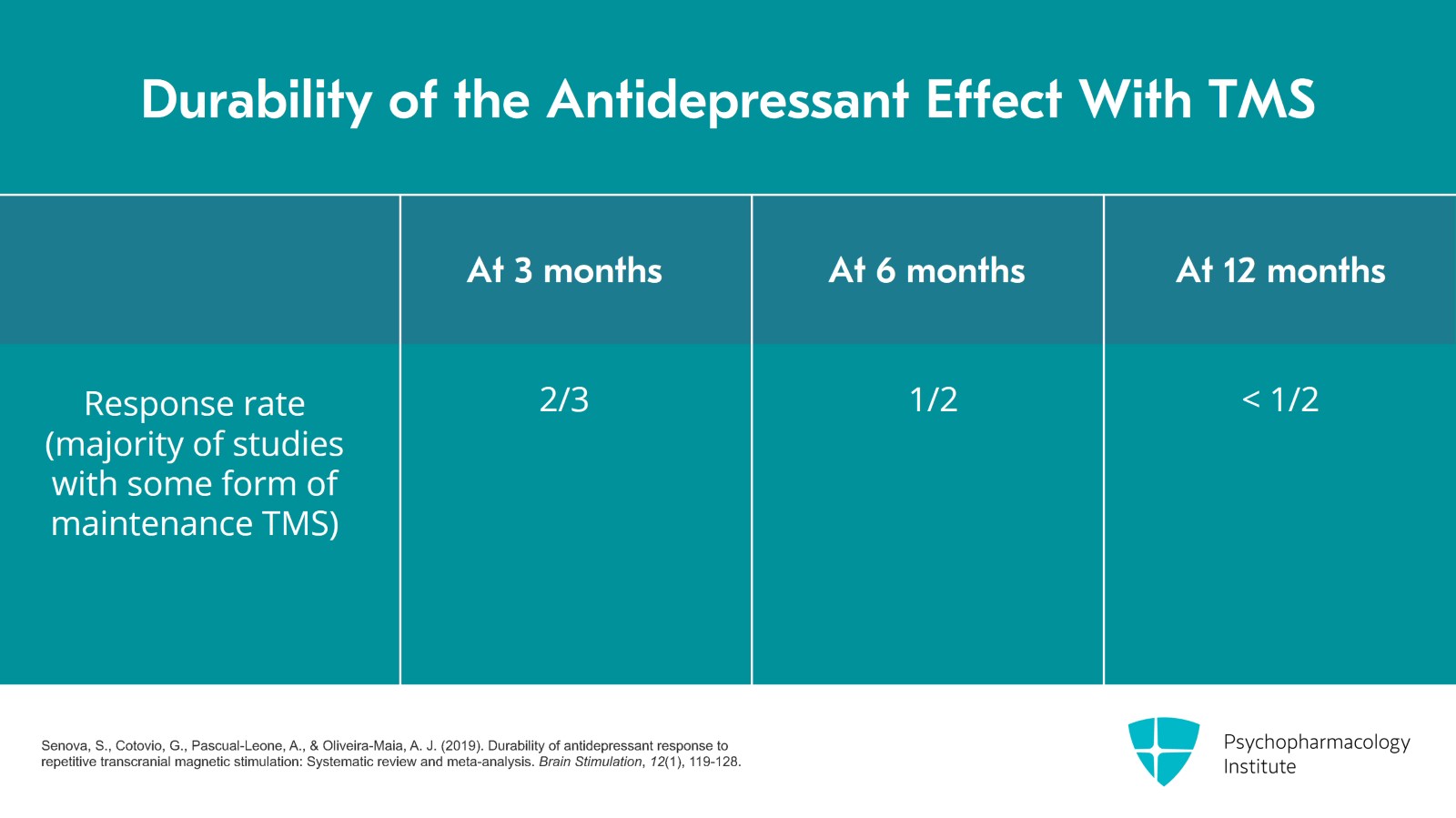
One question that patients will ask is about the durability or relapse rate for TMS. A systematic review published in 2019 answered that question. It included 18 studies between 2002 and 2018 which reported depression outcomes three or more months after rTMS. The majority of these studies, at least 11, had some form of maintenance TMS. The systematic review results are shown here. For patients who responded to the acute course of TMS at three months, 2/3 still maintained the response; at six months, it was about half; and at 12 months, it was a little less than half.
References:
- Senova, S., Cotovio, G., Pascual-Leone, A., & Oliveira-Maia, A. J. (2019). Durability of antidepressant response to repetitive transcranial magnetic stimulation: Systematic review and meta-analysis. Brain Stimulation, 12(1), 119-128.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 27 of 30
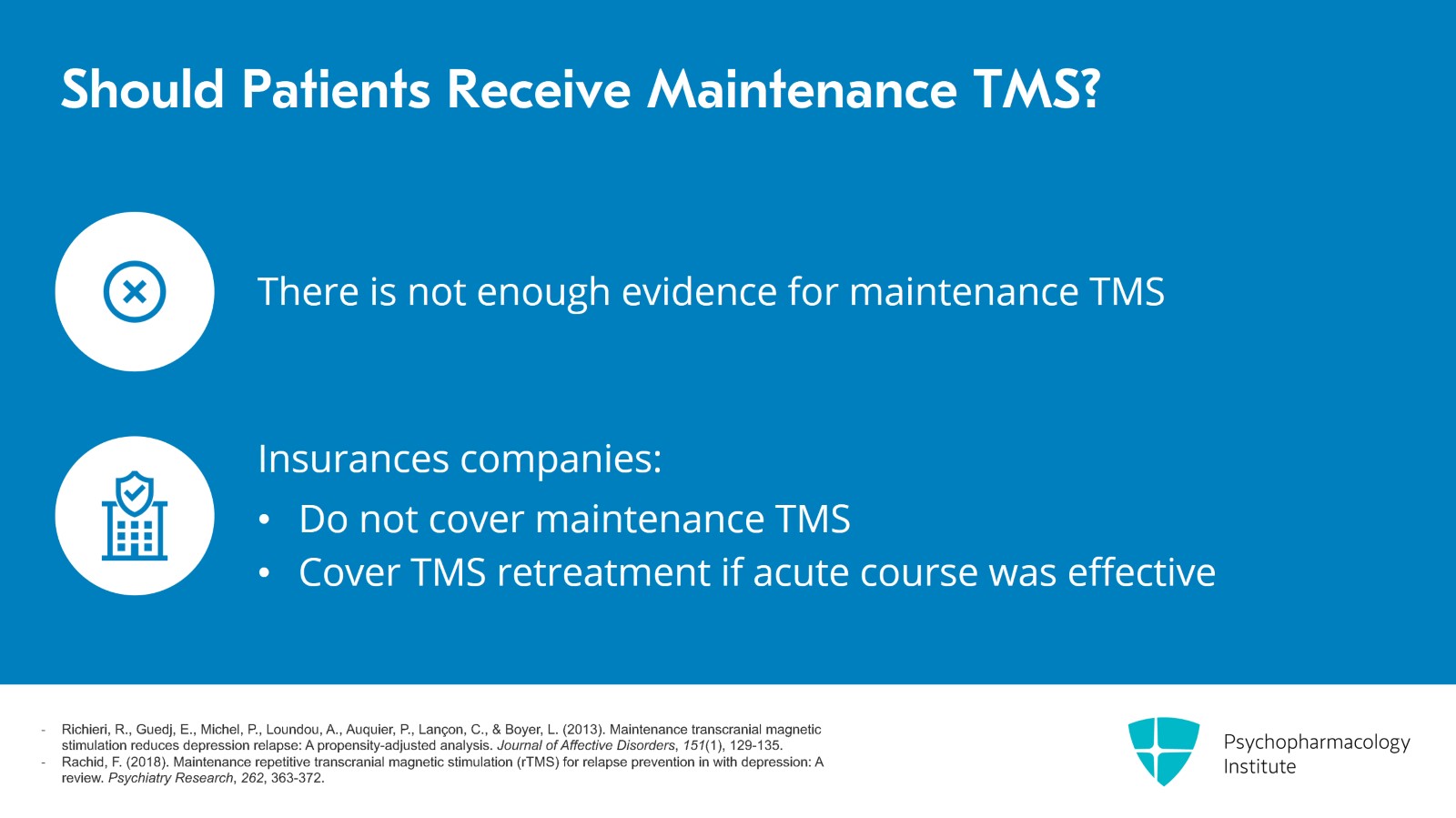
In my clinical practice, I tell patients that the response rate of TMS is about 50% at six months, which brings us to another good question: Should patients get maintenance TMS? There's a small amount of evidence that maintenance TMS can help but not enough evidence for insurance companies to cover maintenance TMS. So practically from a cost standpoint, patients won't get maintenance TMS but pretty much all insurance companies will cover re-treatment with TMS if the patient's mood holds up for at least three months after the acute course TMS.
References:
- Richieri, R., Guedj, E., Michel, P., Loundou, A., Auquier, P., Lançon, C., & Boyer, L. (2013). Maintenance transcranial magnetic stimulation reduces depression relapse: A propensity-adjusted analysis. Journal of Affective Disorders, 151(1), 129-135.
- Rachid, F. (2018). Maintenance repetitive transcranial magnetic stimulation (rTMS) for relapse prevention in with depression: A review. Psychiatry Research, 262, 363-372.
Slide 28 of 30
Key points. In randomized trials, the response rate for active versus sham is not impressive, about 25% versus 11%, but it is about twice as high. Open-label or naturalistic studies show up to 70% response rate. About 60% seems to be a realistic number to tell patients.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.