Text version
Do Antidepressant Side Effects Improve Over Time?
After starting an SSRI two weeks ago, the patient tells you they had the recent onset of insomnia, dry mouth, or loss of libido.
As clinicians, we might refer to the traditional belief that these side effects will improve with medication persistence.
But what is the evidence base to support this belief?
Download PDF and other files
STAR*D Data Offers New Insights
Luckily, a new study attempts to answer this question. It also aims to resolve differences in previous studies with an improved methodology. The authors analyzed results from one of the canons of Psychiatry, the STAR*D trial.
If you recall, the first phase involved starting citalopram at 20 mg q.d. Dose increases went to 40 mg by week 4. The top possible dose was 60 mg in six weeks.
Measuring Side Effects In STAR*D
STAR*D included two rating scales of side effects. The first is the Patient-Rated Inventory of Side Effects or PRISE. It categorizes side effects into nine different organ or function systems.
Within each system, subjects rated their side effects on a 2-point scale:
- 0 as absent
- 1 as tolerable
- 2 as distressing
Rating scales were completed after 2, 4, 6, 9, and 12 weeks. 12 weeks represented a full course of treatment.
Download PDF and other files
Simple Analysis Shows Decreasing Side Effects
The authors included a table of side effects of all participants at each visit. The percentage of subjects reporting each side effect seemed to decrease monotonically from week 2 to week 12. This was particularly true for the more common side effects.
For example, 57% of subjects endorsed difficulty sleeping at week 2. This decreased to 38% by week 12. A similar monotonic decline was seen for other common side effects:
- Headache
- Fatigue
- Anxiety
- Dry mouth
- Loss of sexual desire
Why Simple Analysis Is Misleading
The authors argued that evidence presented this way is not convincing. It doesn’t account for subjects with more distressing side effects dropping out early. This leaves the remaining study group with a lower apparent incidence of side effects.
Download PDF and other files
Improved Analysis Accounts For Dropouts
Here is where the authors improved the methodology over previous studies. They accounted for the likely higher percentage of side effects in subjects leaving the study early. They stratified groups based on their last visit.
They analyzed data on overall side effects from the other rating scale in STAR*D. This was the Frequency, Intensity, and Severity of Side Effects Rating (FISER) and global rating of side effects burden.
Subjects rated overall side effects in the past week in three domains: frequency, intensity, and burden on daily functioning, using a 7-point scale.
Controlling For Depression Symptoms
Many antidepressant side effects overlap or interact with major depression symptoms. The authors created a model controlling for depression severity. They used responses from the Quick Inventory of Depression Symptomatology Self-Rating Scale (QIDS-SR).
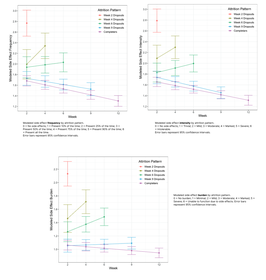
The authors created three colorful graphs. These showed side effects by group over each visit for frequency, intensity, and burden.
Download PDF and other files
Refined Analysis: Multiple Temporal Patterns
Here’s a walk-through of each group:
- Red Band (Dropout at 2 Weeks): Highest side effect scores. Nearly 50% of the time, side effects were of moderate intensity.
- Yellow Band (Dropout at 4 Weeks): Significant increases in both frequency and burden of side effects.
- Green Band (Dropout at 6 Weeks): No significant change in frequency or intensity, but burden increased.
- Blue Band (Dropout at 9 Weeks): First group to show actual improvement in both frequency and intensity.
- Violet Band (12-Week Completers): Significant reductions in all three domains — frequency, intensity, and burden — even when controlling for depression improvement.
Implications for Clinical Practice
This study provides convincing evidence of multiple temporal patterns of side effects.
Key takeaways:
- Patients with troubling side effects at first follow-up may see progressive worsening
- Those completing 9-12 weeks often see overall improvement in side effects
- Side effects don’t completely disappear, even after 12 weeks
Persistent side effects after 12 weeks:
- Sexual side effects were the most distressing:
- 26% reported loss of libido
- 19% had anorgasmia
- 7.2% had erectile difficulties
- 36% of those with sexual side effects found them distressing
- Sleep-related side effects were next:
- 38% had difficulty sleeping
- 18% reported hypersomnia
- 30% found their sleep-related side effects distressing
So, even in treatment completers, substantial side effects persisted — especially sexual and sleep-related symptoms.
Download PDF and other files
Conclusion: Assess Early
This study highlights the importance of assessing side effects at the first follow-up visit after starting an antidepressant.
For patients with tolerable side effects who wish to complete treatment, we can reassure them that evidence suggests their side effects may improve over time. However, it’s crucial to acknowledge that some side effects may persist even after a full trial.
Download PDF and other files
Abstract
Not all types of depressed patients who persist with their antidepressant treatment improve in side effect complaints: A comparison of treatment completers and dropouts in the STAR*D trial
Thomas T Kim, M.D. & Colin Xu, M.D.
Introduction: There is a “traditional belief” that antidepressant side effect complaints improve with medication persistence; however, support for this theory has remained inconclusive. We aimed to examine if side effect complaints improved over time by modeling the relationship between side effect complaints and time at dropout for patients receiving citalopram during the first level of acute treatment in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial.
Methods: We categorized the 2833 patients into five patterns by week of dropout. We used pattern-mixture modeling to model change in side effect complaints (frequency, intensity, and burden) over the 12-week course of treatment, while accounting for attrition and depressive severity. Using post-hoc linear contrasts, we compared the attrition patterns with the completers’ pattern for severity of side effect complaints at each respective last visit prior to dropout as well as averaged side effect complaints across the duration of treatment. We also reported frequencies and tolerability of side effects for nine organ/function systems over the course of treatment.
Results: Patients who dropped out early exhibited worsening side effect burden and patients who dropped out later showed improvements in side effect frequency and intensity. Treatment completers improved in all side effect complaints over the course of treatment. Early attrition patterns had more severe side effect complaints for both tests of post-hoc linear contrasts than later attrition patterns and completers.
Conclusions: Side effect complaints from antidepressant treatment improve over time, but only for some types of patients. As a precaution for early dropout, clinicians should monitor patients who exhibit worsening and more severe side effect complaints-especially in the first 6 weeks of antidepressant treatment. In addition, clinicians may want to consider changing the type of treatment early on for these patients, rather than encouraging them to persist with their current medication.
Keywords: SSRI side effect symptoms; SSRI side effect tolerability; STAR*D; antidepressant persistence.
Reference
Kim, T. M.D. & Xu, C. M.D. (2025). Not all types of depressed patients who persist with their antidepressant treatment improve in side effect complaints: A comparison of treatment completers and dropouts in the STAR*D trial. Acta Psychiatrica Scandinavica;151(2):152-162. Epub 2024 Oct 3.



