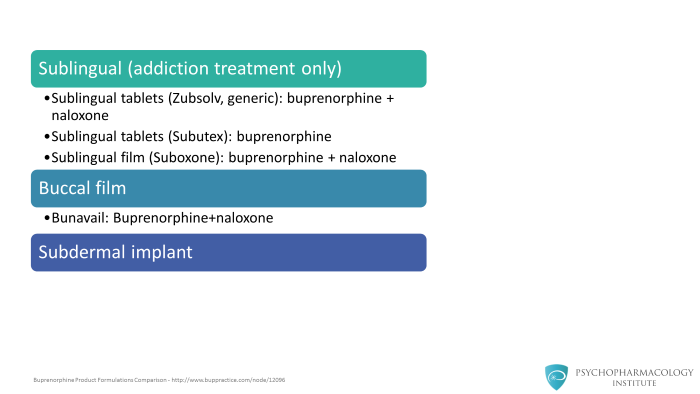
Buprenorphine comes in various formulations.
It is available as a sublingual formulation in both tablets and films. These formulations are used exclusively for addiction treatment in the United States.
The sublingual formulation comes in two different versions, either as a combination tablet of buprenorphine and naloxone or simply just buprenorphine.
In addition, there are buccal film formulations. Now there is also a buprenorphine implant available.
Research is now ongoing for various kinds of implant formulations. But for the most part currently, the most widely used formulations of buprenorphine for addiction treatment are the sublingual formulations, both tablets and films.
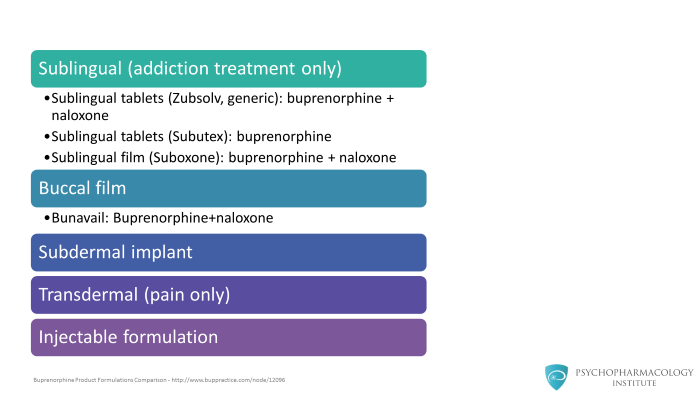
There are other buprenorphine formulations that are available for chronic pain treatment. These are transdermal formulations or injectable formulations as well as buccal formulations.
In the United States, only the sublingual formulations or the implants are approved for the treatment of opioid addiction or opioid use disorders. The other formulations, the transdermal patch, et cetera, are approved for chronic pain treatment.

If the physician wants to prescribe sublingual formulations for addiction treatment, the physician must have the X waiver or the DEA waiver.
The reason why the sublingual formulations come in two formulations is that the combination tablet contains naloxone and this combination tablet is a default choice for the vast majority of the patients in treatment. The naloxone is included in this product because it will discourage the injection misuse of the medication.
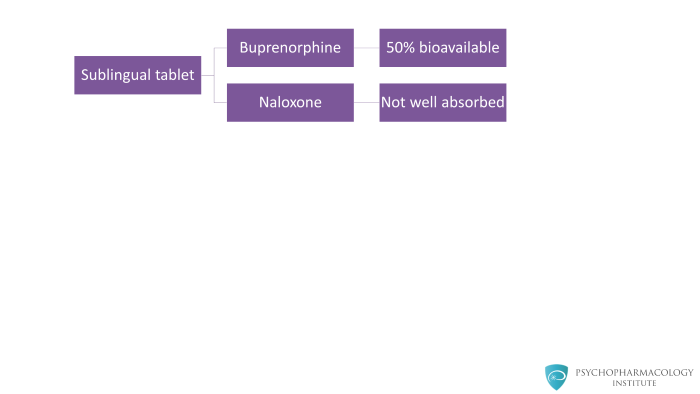
When the tablet is taken as prescribed, meaning sublingually, buprenorphine is about 50 percent bioavailable. The naloxone has extremely poor bioavailability potentially in single digits or even less.
Sublingually taken, buprenorphine is well absorbed but naloxone is not well absorbed at all. Therefore, the net effect is that you get the effect of buprenorphine but naloxone is not active.
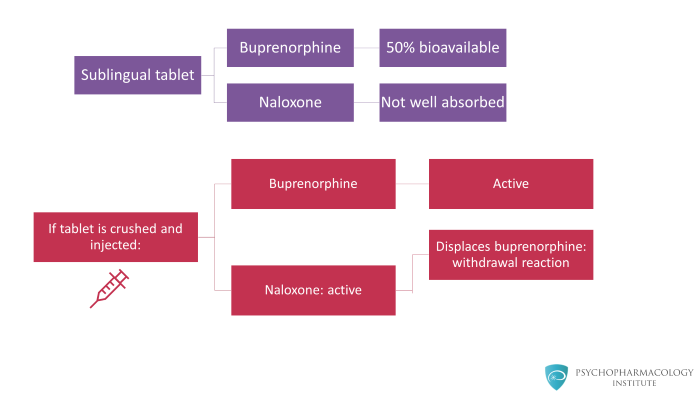
However, if the product is injected, crushed and injected, then both buprenorphine and naloxone are active. And the naloxone will displace the buprenorphine producing a short lived, maybe 60 to 90 minutes of an uncomfortable withdrawal reaction.
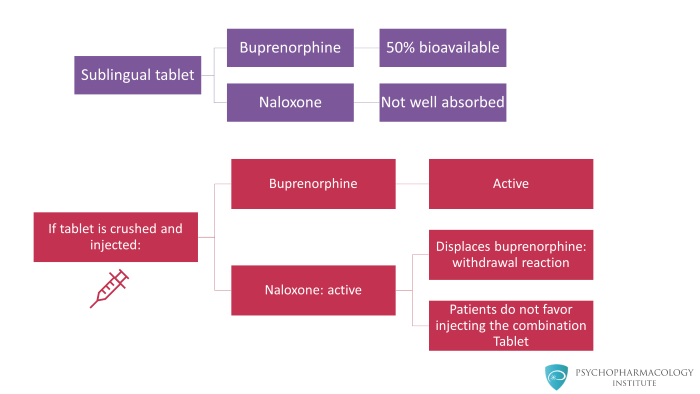
Therefore, studies have well documented that if individuals try injecting the combination tablet, they get a very dysphoric reaction that is a mild to moderate withdrawal effect. Generally speaking, patients do not like this.
Studies have confirmed that patients generally do not favor injecting the combination tablet. Therefore, clinicians are recommended to use only the combination tablet for the vast majority of the patients.
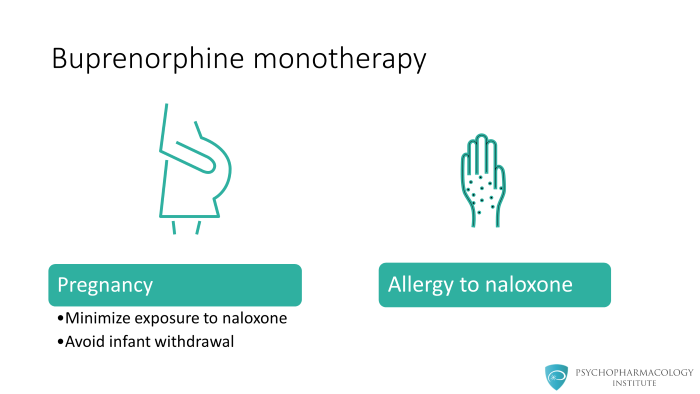
The mono tablet is used currently only for two types of patients:
1 – Pregnant patients, where we want to minimize exposure to other substances like naloxone or inadvertently produce withdrawal in the infant. Therefore, for pregnant patients who are on treatment, we generally use only the mono tablet.
2 – If the patient has a confirmed allergy to naloxone. This does happen occasionally. And for those patients, the combination tablet should not be used, only the mono tablet should be used.
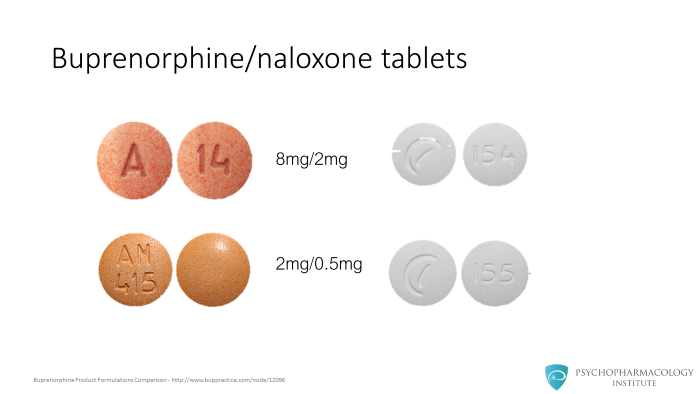
Both of these formulations (combination and mono tablet) come in two sizes, the 8/2 mg or the 2/0.5 mg. The first number refers to the buprenorphine dose. The second number refers to the naloxone dose. It comes in a 4:1 ratio.

The sublingual films come in additional different sizes whether buprenorphine dose comes in 2, 4, 8 or 12 mg doses with the corresponding naloxone dose at the 4:1 ratio.
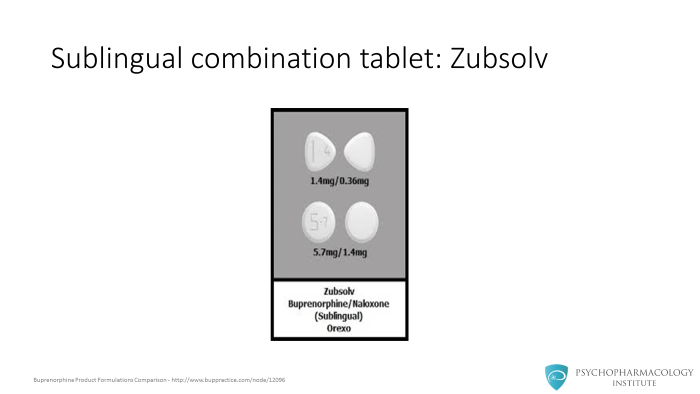
There is an additional brand sublingual tablet called Zubsolv which only comes in the combination tablet with a higher bioavailability leading to smaller dose ranges. And finally, a buccal film formulation also is available for addiction treatment.
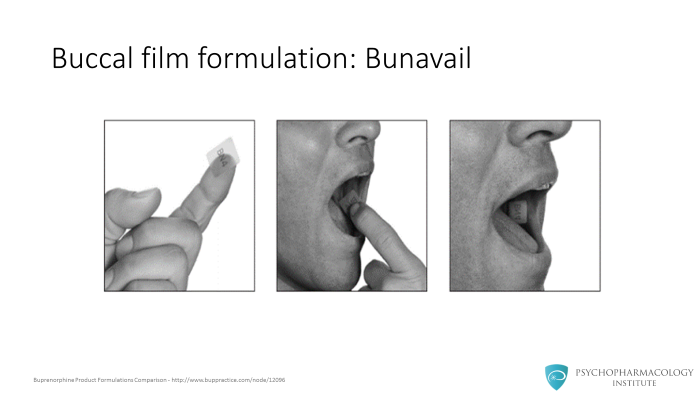
And finally, a buccal film formulation also is available for addiction treatment.
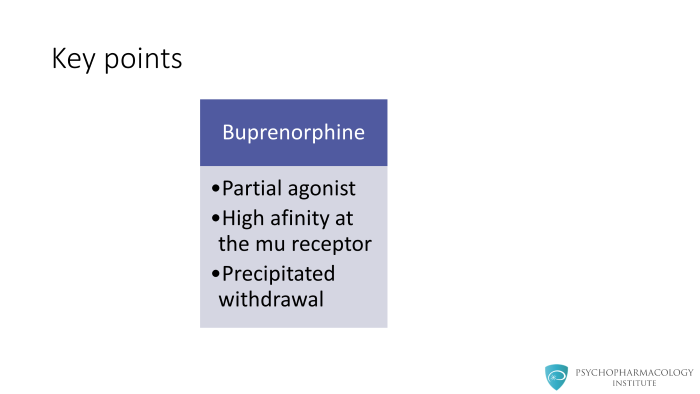
Key Points Buprenorphine is a partial agonist with a high affinity at the mu receptor leading to the possibility for a precipitated withdrawal if opioids are still on the opioid receptors.
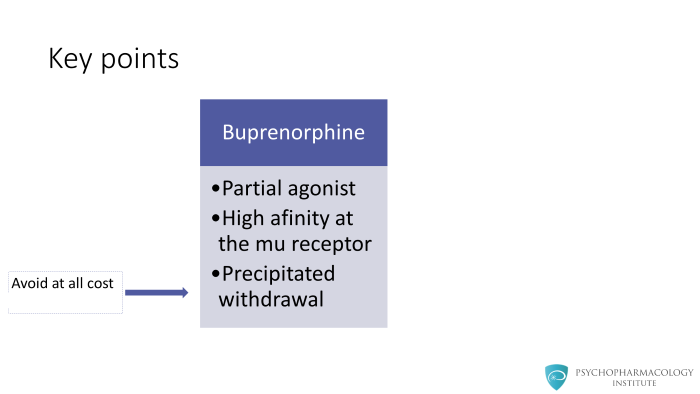
This precipitated withdrawal is the most concerning adverse effect of buprenorphine treatment and should be avoided at all cost.

The best way to do this is to ensure that patients are already in mild opioid withdrawal.

The combination tablet that contains both buprenorphine and naloxone is the default choice for the vast majority of opioid addicted patients.
References
- Buprenorphine Product Formulations Comparison – http://www.buppractice.com/node/12096



