Slides and Transcript
Slide 2 of 22
To date, no serious adverse events have been attributed to either of the broad-spectrum micronutrient formulas.
References:
- Simpson, J. S., Crawford, S. G., Goldstein, E. T., Field, C., Burgess, E., & Kaplan, B. J. (2011). Systematic review of safety and tolerability of a complex micronutrient formula used in mental health. BMC Psychiatry, 11(1).
- Gerbarg, P. L., Muskin, P. R., & Brown, R. P. (2017). Complementary and integrative treatments in psychiatric practice. American Psychiatric Pub.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 3 of 22
Biological safety data from 144 children and adults in a paper published in 2011 showed no occurrences of clinically meaningful negative effects or abnormal blood tests that could be attributed to toxicity. Adverse events were only minor and these were commonly transitory reports of headaches and nausea.
References:
- Simpson, J. S., Crawford, S. G., Goldstein, E. T., Field, C., Burgess, E., & Kaplan, B. J. (2011). Systematic review of safety and tolerability of a complex micronutrient formula used in mental health. BMC Psychiatry, 11(1).
- Rucklidge, J. J., Eggleston, M., Johnstone, J. M., Darling, K., & Frampton, C. M. (2018). Vitamin-mineral treatment improves aggression and emotional regulation in children with ADHD: A fully blinded, randomized, placebo-controlled trial. Journal of Child Psychology and Psychiatry, 59(3), 232–246.
- Rucklidge, J. J., Frampton, C. M., Gorman, B., & Boggis, A. (2014). Vitamin–mineral treatment of attention-deficit hyperactivity disorder in adults: Double-blind randomised placebo-controlled trial. British Journal of Psychiatry, 204(4), 306-315.
- Johnstone, J. M., Hatsu, I., Tost, G., Srikanth, P., Eiterman, L. P., Bruton, A. M., Ast, H. K., Robinette, L. M., Stern, M. M., Millington, E. G., Gracious, B. L., Hughes, A. J., Leung, B. M. Y., & Arnold, L. E. (2022). Micronutrients for attention-deficit/hyperactivity disorder in youths: A placebo-controlled randomized clinical trial. Journal of the American Academy of Child and Adolescent Psychiatry, 61(5), 647–661.
Slide 4 of 22
Only one of the studies permitted a direct comparison between broad-spectrum micronutrient treatment and medication. This was a study comparing micronutrients to psychiatric medication for the treatment of autism-related symptoms. The group treated with medication had 6.5 times as many adverse events and more weight gain compared to the group treated with micronutrients.
References:
- Mehl-Madrona, L., Leung, B., Kennedy, C., Paul, S., & Kaplan, B. J. (2010). Micronutrients versus standard medication management in autism: A naturalistic case-control study. Journal of Child and Adolescent Psychopharmacology, 20(2), 95–103.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 5 of 22
Now, let's discuss what we know about the long-term use of micronutrients. In this study of 34 adults and children who'd been taking micronutrients for an average of 2.66 years, CBC, LFTs, kidney function test, coagulation profiles, fasting glucose, iron studies, key nutrients, and prolactin were tested.
References:
- Rucklidge, J. J., Eggleston, M. J., Ealam, B., Beaglehole, B., & Mulder, R. T. (2019). An observational preliminary study on the safety of long-term consumption of micronutrients for the treatment of psychiatric symptoms. The Journal of Alternative and Complementary Medicine, 25(6), 613-622.
Slide 6 of 22
Excluding B12 which could be expected to be high, 94.6% were within test reference ranges and those outside the reference ranges were mostly just outside reference ranges. One participant was diagnosed with hemochromatosis based on iron studies. No other clinically significant adverse effects were reported.
References:
- Rucklidge, J. J., Eggleston, M. J., Ealam, B., Beaglehole, B., & Mulder, R. T. (2019). An observational preliminary study on the safety of long-term consumption of micronutrients for the treatment of psychiatric symptoms. The Journal of Alternative and Complementary Medicine, 25(6), 613-622.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 7 of 22
The authors of the article on long-term use of micronutrients concluded, overall, the substantial psychiatric benefits observed appear to outweigh the minimal observed risks in these participants.
References:
- Rucklidge, J. J., Eggleston, M. J., Ealam, B., Beaglehole, B., & Mulder, R. T. (2019). An observational preliminary study on the safety of long-term consumption of micronutrients for the treatment of psychiatric symptoms. The Journal of Alternative and Complementary Medicine, 25(6), 613-622.
Slide 8 of 22
Larger long-term studies are needed. However, to date, funding for micronutrient research has been limited. Only through large sampling and post-marketing surveillance is it possible to identify less common adverse effects associated with this treatment.
References:
- Rucklidge, J. J., Eggleston, M. J., Ealam, B., Beaglehole, B., & Mulder, R. T. (2019). An observational preliminary study on the safety of long-term consumption of micronutrients for the treatment of psychiatric symptoms. The Journal of Alternative and Complementary Medicine, 25(6), 613-622.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 9 of 22
Now, let's discuss laboratory testing. The current research has shown no significant changes in standard blood or urine measures. Laboratory testing in the adult ADHD RCT did show an uncommon, asymptomatic, clinically insignificant increase in prolactin levels which was within normal limits in all cases. This increase was not observed in the child ADHD RCTs.
References:
- Rucklidge, J. J., Eggleston, M. J., Ealam, B., Beaglehole, B., & Mulder, R. T. (2019). An observational preliminary study on the safety of long-term consumption of micronutrients for the treatment of psychiatric symptoms. The Journal of Alternative and Complementary Medicine, 25(6), 613-622.
Slide 10 of 22
Screening for potential medical problems is recommended before initiating treatment.
References:
- Rucklidge, J. J., Eggleston, M. J., Ealam, B., Beaglehole, B., & Mulder, R. T. (2019). An observational preliminary study on the safety of long-term consumption of micronutrients for the treatment of psychiatric symptoms. The Journal of Alternative and Complementary Medicine, 25(6), 613-622.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 11 of 22
The authors noted that although hemochromatosis is rare, given they did find a patient with it in their cohort, it seems reasonable to recommend screening for metabolic and electrolyte abnormalities before starting treatment to prevent similar future occurrences and possible harm. They suggested that pretreatment screening should include CBC, CMP, calcium, phos, mag, copper, ceruloplasmin, vitamin B12, folate and iron studies.
References:
- Rucklidge, J. J., Eggleston, M. J., Ealam, B., Beaglehole, B., & Mulder, R. T. (2019). An observational preliminary study on the safety of long-term consumption of micronutrients for the treatment of psychiatric symptoms. The Journal of Alternative and Complementary Medicine, 25(6), 613-622.
Slide 12 of 22
Long-term pharmacovigilance monitoring is required to ascertain any rare but significant adverse effects. In the absence of large studies with long-term use, it may be prudent to do occasional follow-up laboratory testing.
References:
- Rucklidge, J. J., Eggleston, M. J., Ealam, B., Beaglehole, B., & Mulder, R. T. (2019). An observational preliminary study on the safety of long-term consumption of micronutrients for the treatment of psychiatric symptoms. The Journal of Alternative and Complementary Medicine, 25(6), 613-622.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 13 of 22
For those with iron deficiency, the formulas are likely not sufficient to fully replete as the product contains very little iron. The products do contain enough B12 and folate for most individuals with mild deficiencies. The formula label notes how much of each nutrient is in the formula and can be referenced to see if additional repletion is necessary for those low in those specific nutrients.
References:
- Popper C. W. (2014). Single-micronutrient and broad-spectrum micronutrient approaches for treating mood disorders in youth and adults. Child and Adolescent Psychiatric Clinics of North America, 23(3), 591–672.
- Gerbarg, P. L., Muskin, P. R., & Brown, R. P. (2017). Complementary and integrative treatments in psychiatric practice. American Psychiatric Pub.
Slide 14 of 22
What are some potential side effects of micronutrients? Okay, well, before we delve into this, it's important to note that in all the RCTs, these minor side effects have occurred equally in both the micronutrient and placebo groups. Side effects were generally mild.
References:
- Popper C. W. (2014). Single-micronutrient and broad-spectrum micronutrient approaches for treating mood disorders in youth and adults. Child and Adolescent Psychiatric Clinics of North America, 23(3), 591–672.
- Gerbarg, P. L., Muskin, P. R., & Brown, R. P. (2017). Complementary and integrative treatments in psychiatric practice. American Psychiatric Pub.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 15 of 22
Side effects included transient headache which improved with hydration; insomnia which is not surprising given B vitamins can be activating for some individuals; loose stools which were typically transient as well as nausea, dyspepsia, flatulence.
References:
- Popper C. W. (2014). Single-micronutrient and broad-spectrum micronutrient approaches for treating mood disorders in youth and adults. Child and Adolescent Psychiatric Clinics of North America, 23(3), 591–672.
- Gerbarg, P. L., Muskin, P. R., & Brown, R. P. (2017). Complementary and integrative treatments in psychiatric practice. American Psychiatric Pub.
Slide 16 of 22
From a clinical perspective, GI side effects are often worse when combined with other treatments with GI side effect propensity or those with preexisting GI dysfunction.
References:
- Popper C. W. (2014). Single-micronutrient and broad-spectrum micronutrient approaches for treating mood disorders in youth and adults. Child and Adolescent Psychiatric Clinics of North America, 23(3), 591–672.
- Gerbarg, P. L., Muskin, P. R., & Brown, R. P. (2017). Complementary and integrative treatments in psychiatric practice. American Psychiatric Pub.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 17 of 22
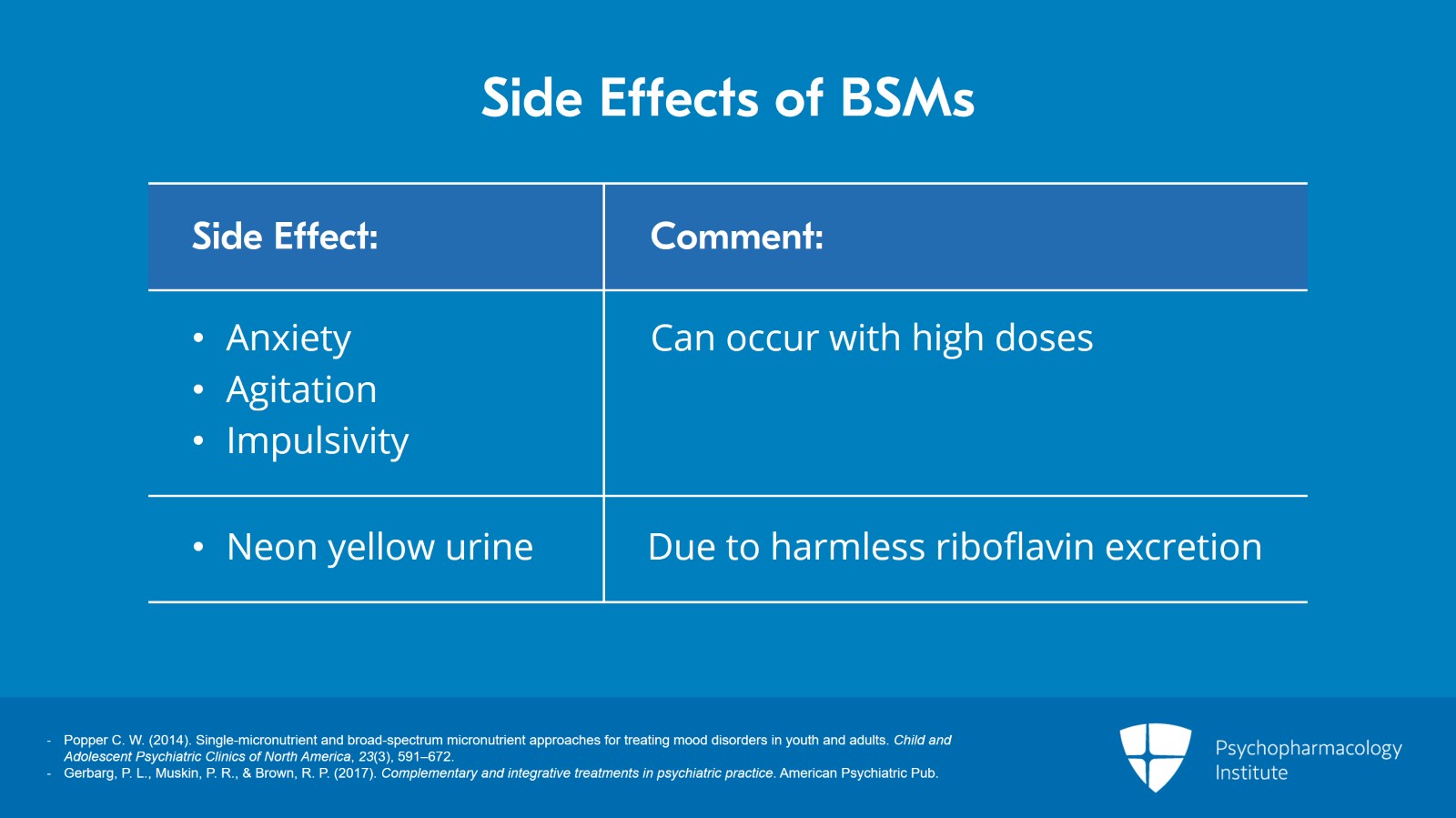
Anxiety, agitation or impulsivity can occur if the dose is too high and lowering the dose tends to reduce agitation for many. Lastly, the urine can change to a neon yellow color, secondary to harmless riboflavin excretion with micronutrients.
References:
- Popper C. W. (2014). Single-micronutrient and broad-spectrum micronutrient approaches for treating mood disorders in youth and adults. Child and Adolescent Psychiatric Clinics of North America, 23(3), 591–672.
- Gerbarg, P. L., Muskin, P. R., & Brown, R. P. (2017). Complementary and integrative treatments in psychiatric practice. American Psychiatric Pub.
Slide 18 of 22
The World Federation of Societies of Biological Psychiatry and the Canadian Network for Mood and Anxiety Treatments task force developed clinician guidelines for the treatment of psychiatric disorders with nutraceuticals and phytoceuticals and concluded that micronutrients had an evidence grade of A given the two RCTs that had been published at the time of the writing of the guidelines as well as the meta-analysis showing supportive evidence of efficacy in ADHD as monotherapy, one in children and one in adults. They rated the strength of recommendation for micronutrients as weakly recommend for ADHD. They noted that this particular micronutrient formula's efficacy cannot be extended to other micronutrient formulas and rated the safety data for micronutrients as acceptable.
References:
- Sarris, J., Ravindran, A., Yatham, L. N., Marx, W., Rucklidge, J. J., McIntyre, R. S., Akhondzadeh, S., Benedetti, F., Caneo, C., Cramer, H., Cribb, L., De Manincor, M., Dean, O., Deslandes, A. C., Freeman, M. P., Gangadhar, B., Harvey, B. H., Kasper, S., Lake, J., … Berk, M. (2022). Clinician guidelines for the treatment of psychiatric disorders with nutraceuticals and phytoceuticals: The world Federation of Societies of Biological Psychiatry (WFSBP) and Canadian network for mood and anxiety treatments (CANMAT) Taskforce. The World Journal of Biological Psychiatry, 23(6), 424-455.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 19 of 22
Key points: Safety and tolerability data have been evaluated in several RCTs and to date, there have been no reported severe adverse events attributed to these two formulas of broad-spectrum micronutrients.
Slide 20 of 22
Side effects are typically mild and include nausea, headache, insomnia, GI symptoms, anxiety, and neon yellow urine. In the absence of large studies with long-term use, researchers have suggested baseline and follow-up laboratory testing.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 21 of 22
The World Federation of Societies of Biological Psychiatry and Canadian Network for Mood and Anxiety Treatments task force have weakly recommended the use of broad-spectrum micronutrients, rate the safety data as acceptable, and data for evidence as grade A.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.