Slides and Transcript
Slide 1 of 14
Now, let’s talk about tailoring the medication choices for alcohol use disorder treatment, which is probably top of mind of most. You probably were all aware of the medications we use. But which ones do you use?
Slide 2 of 14
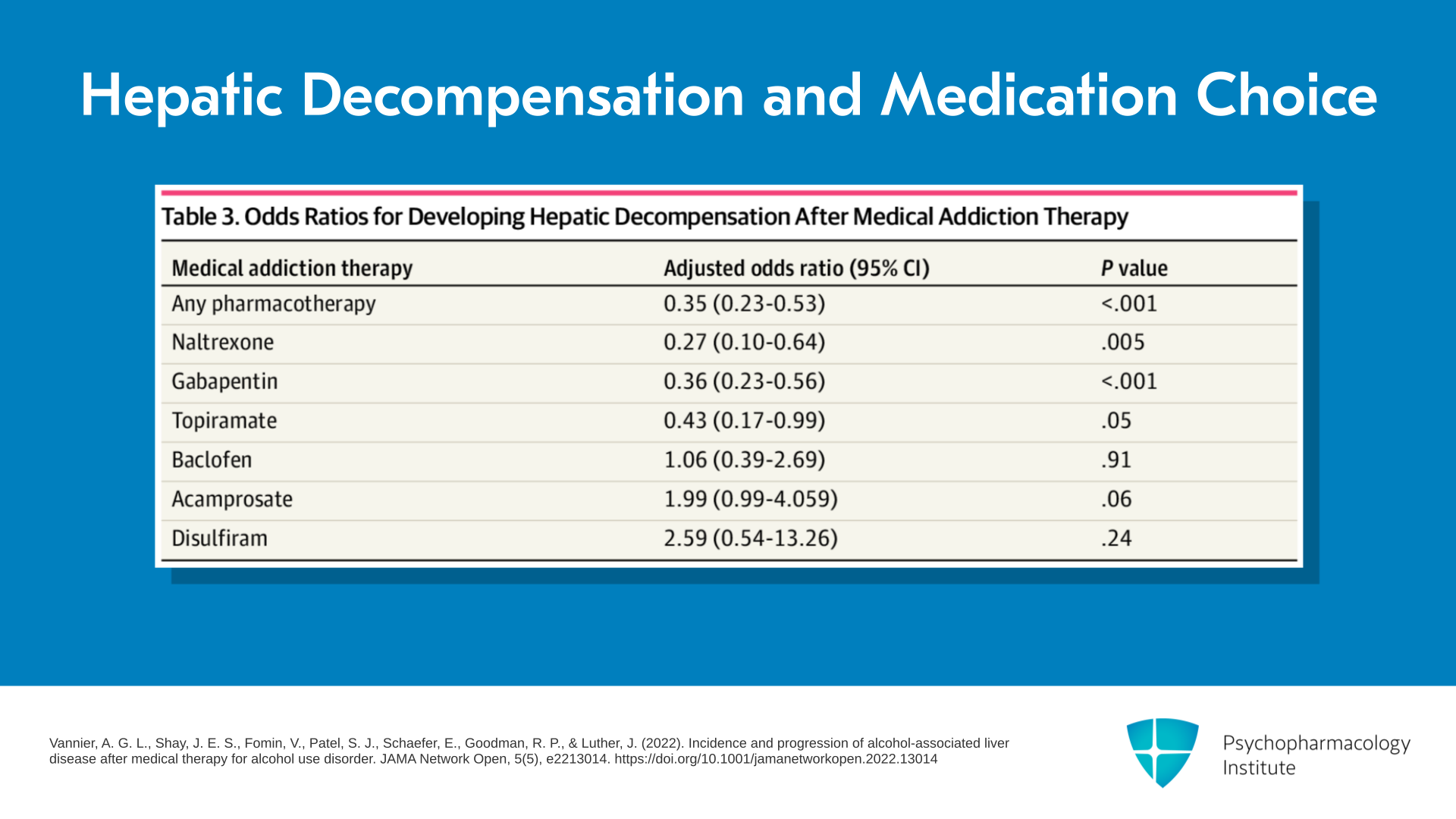
Well, first of all, one of the major drivers is whether there’s the presence of hepatic decompensation or not. So again, I refer to this article by Vannier et al. in 2022. There’s a table in there which I think is wonderful. It shows the odds ratio of developing hepatic decompensation after you start MAUD. And it shows as I try to convince you in the very first section of this presentation that any MAUD reduces the odds ratio of going on to hepatic decompensation by about two-thirds. So in other words, the adjusted odds ratio is 0.35 favoring the pharmacotherapy versus no treatment.
References:
- Vannier, A. G. L., Shay, J. E. S., Fomin, V., Patel, S. J., Schaefer, E., Goodman, R. P., & Luther, J. (2022). Incidence and progression of alcohol-associated liver disease after medical therapy for alcohol use disorder. JAMA Network Open, 5(5), e2213014. https://doi.org/10.1001/jamanetworkopen.2022.13014
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 3 of 14
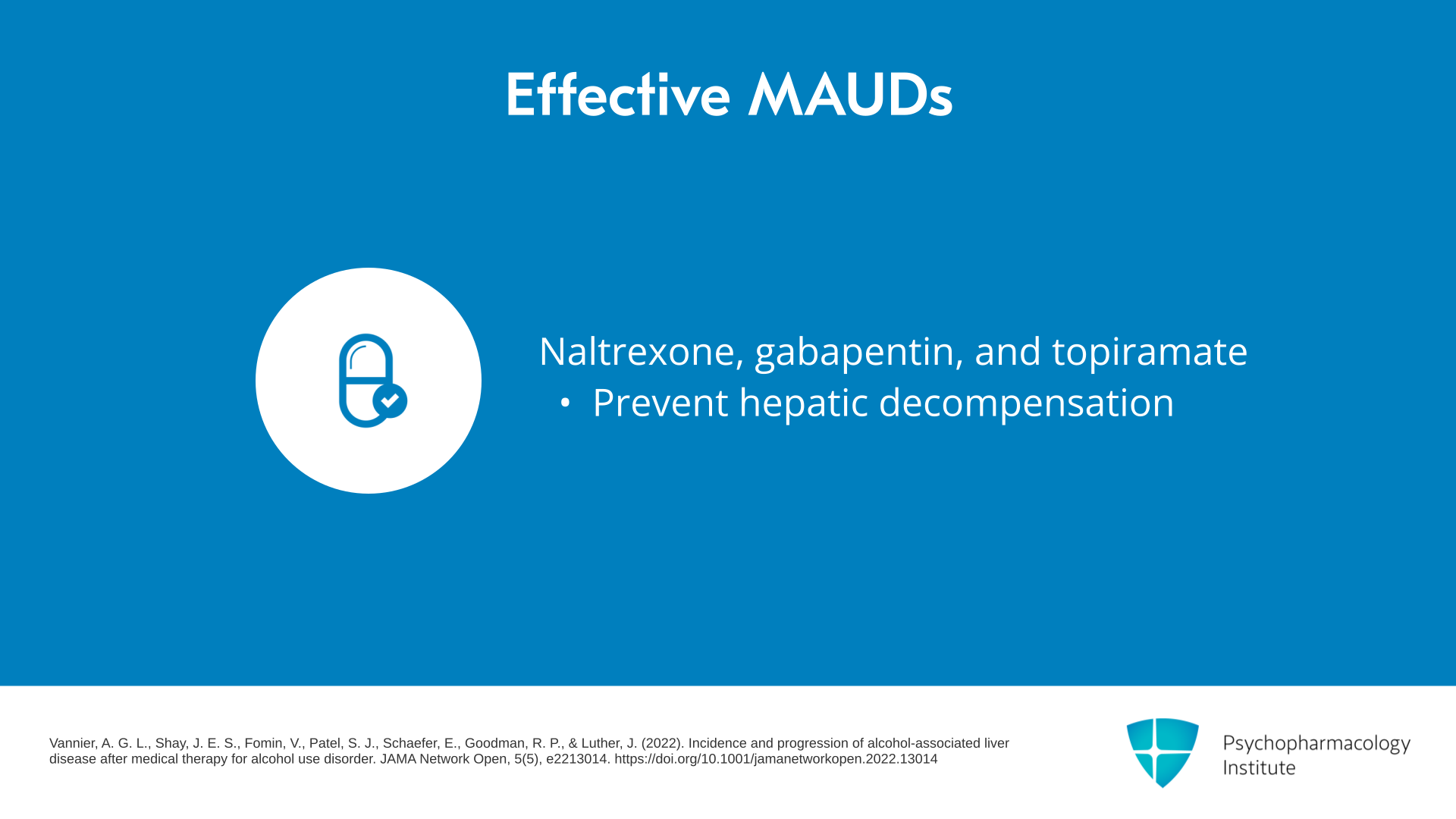
Which ones are the most effective in this Vannier comparison? Naltrexone, gabapentin, and topiramate. They’re all moderately effective – 0.27, 0.36, and 0.43 in effect sizes – for not going on to hepatic decompensation. So that would argue those three are safe even though I would guess most of you would think that naltrexone is not safe for the liver.
References:
- Vannier, A. G. L., Shay, J. E. S., Fomin, V., Patel, S. J., Schaefer, E., Goodman, R. P., & Luther, J. (2022). Incidence and progression of alcohol-associated liver disease after medical therapy for alcohol use disorder. JAMA Network Open, 5(5), e2213014. https://doi.org/10.1001/jamanetworkopen.2022.13014
Slide 4 of 14
But interestingly, baclofen had no effect, and then there were two that actually seemed to do worse in terms of progression to hepatic decompensation – acamprosate had an adjusted odds ratio of 1.99 and disulfiram 2.59, so much worse. But I point out that the p-value, in other words, the significance of the difference, is actually not 0.05 or less. So you need to take that with a bit of a grain of salt. But it supports naltrexone, gabapentin, and topiramate, being quite effective ’cause those were all significant at 0.05 or less.
References:
- Vannier, A. G. L., Shay, J. E. S., Fomin, V., Patel, S. J., Schaefer, E., Goodman, R. P., & Luther, J. (2022). Incidence and progression of alcohol-associated liver disease after medical therapy for alcohol use disorder. JAMA Network Open, 5(5), e2213014. https://doi.org/10.1001/jamanetworkopen.2022.13014
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 5 of 14
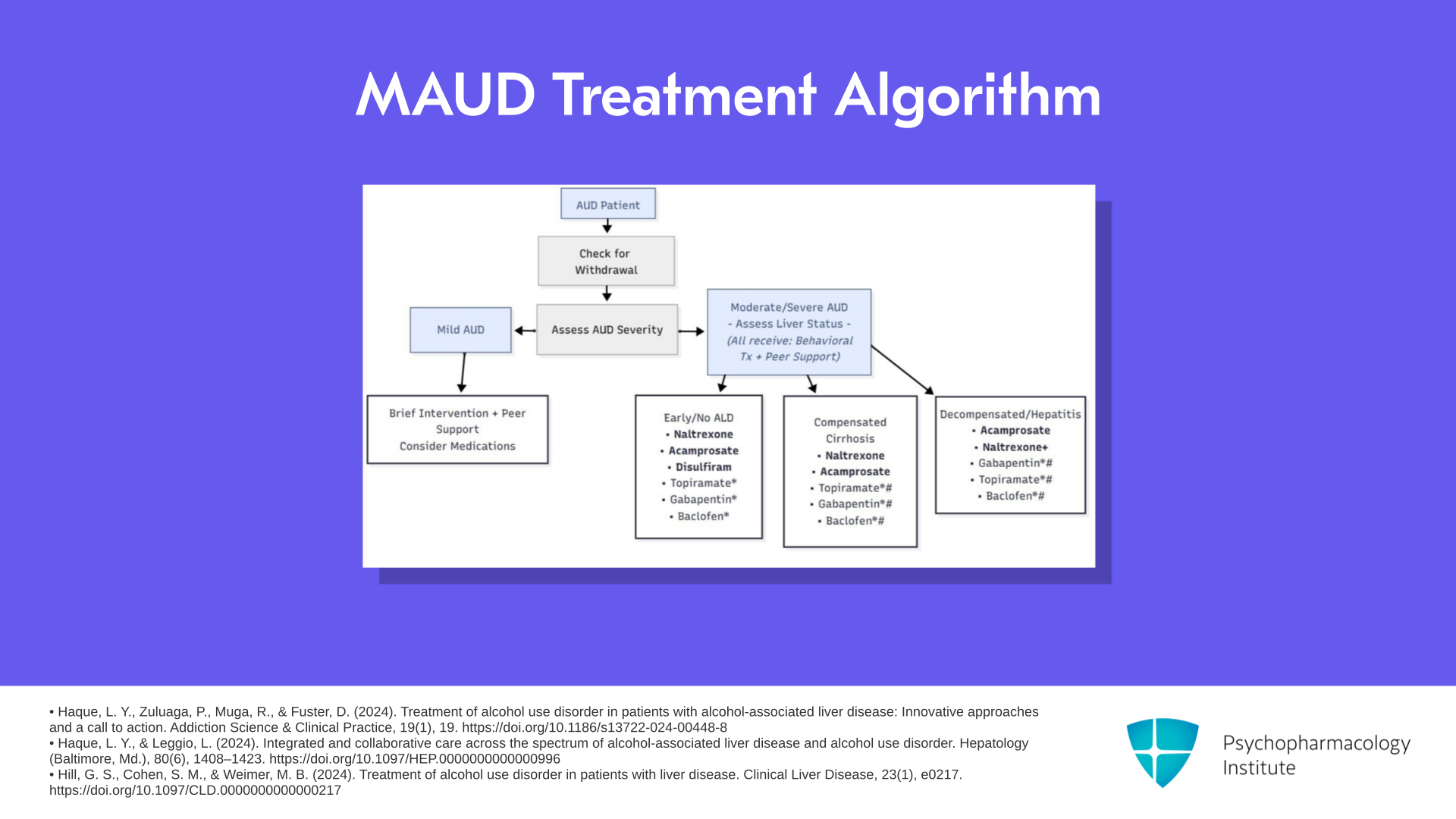
The next slide is something that came from Lamia Haque at Yale University, one of my colleagues, and is somewhat included in a review by Melissa Weimer. So this is a little complicated that’s why I’m going to take a little time to go through it.
References:
- Haque, L. Y., Zuluaga, P., Muga, R., & Fuster, D. (2024). Treatment of alcohol use disorder in patients with alcohol-associated liver disease: Innovative approaches and a call to action. Addiction Science & Clinical Practice, 19(1), 19. https://doi.org/10.1186/s13722-024-00448-8
- Haque, L. Y., & Leggio, L. (2024). Integrated and collaborative care across the spectrum of alcohol-associated liver disease and alcohol use disorder. Hepatology (Baltimore, Md.), 80(6), 1408–1423. https://doi.org/10.1097/HEP.0000000000000996
- Hill, G. S., Cohen, S. M., & Weimer, M. B. (2024). Treatment of alcohol use disorder in patients with liver disease. Clinical Liver Disease, 23(1), e0217. https://doi.org/10.1097/CLD.0000000000000217
Slide 6 of 14
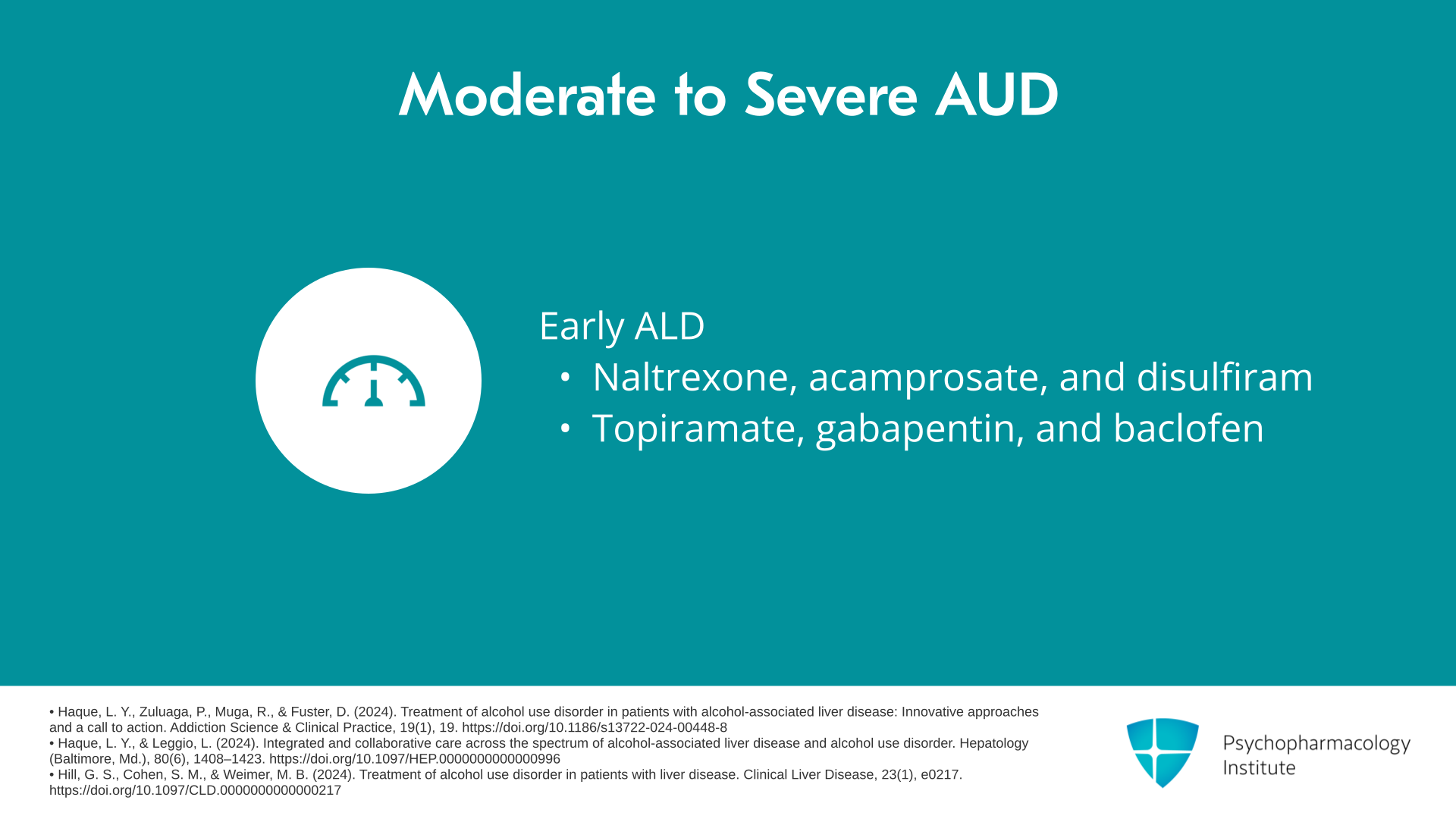
Let’s say you have a patient and if there are withdrawal symptoms of course you do the withdrawal management first, then you go over to decide what medications to use. If they have moderate to severe alcohol use disorder and they’re with early alcoholic liver disease or no alcoholic liver disease, your choices in order are naltrexone, then acamprosate, then disulfiram, and then you can have lesser choices of topiramate, gabapentin, and baclofen. So this algorithm very much favors the use of the FDA-approved medications as long as it’s safe.
References:
- Haque, L. Y., Zuluaga, P., Muga, R., & Fuster, D. (2024). Treatment of alcohol use disorder in patients with alcohol-associated liver disease: Innovative approaches and a call to action. Addiction Science & Clinical Practice, 19(1), 19. https://doi.org/10.1186/s13722-024-00448-8
- Haque, L. Y., & Leggio, L. (2024). Integrated and collaborative care across the spectrum of alcohol-associated liver disease and alcohol use disorder. Hepatology (Baltimore, Md.), 80(6), 1408–1423. https://doi.org/10.1097/HEP.0000000000000996
- Hill, G. S., Cohen, S. M., & Weimer, M. B. (2024). Treatment of alcohol use disorder in patients with liver disease. Clinical Liver Disease, 23(1), e0217. https://doi.org/10.1097/CLD.0000000000000217
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 7 of 14
In the presence of compensated cirrhosis, we take disulfiram away. So the choices are again, in order, naltrexone, acamprosate, and then the non-FDA-approved medications.
References:
- Haque, L. Y., Zuluaga, P., Muga, R., & Fuster, D. (2024). Treatment of alcohol use disorder in patients with alcohol-associated liver disease: Innovative approaches and a call to action. Addiction Science & Clinical Practice, 19(1), 19. https://doi.org/10.1186/s13722-024-00448-8
- Haque, L. Y., & Leggio, L. (2024). Integrated and collaborative care across the spectrum of alcohol-associated liver disease and alcohol use disorder. Hepatology (Baltimore, Md.), 80(6), 1408–1423. https://doi.org/10.1097/HEP.0000000000000996
- Hill, G. S., Cohen, S. M., & Weimer, M. B. (2024). Treatment of alcohol use disorder in patients with liver disease. Clinical Liver Disease, 23(1), e0217. https://doi.org/10.1097/CLD.0000000000000217
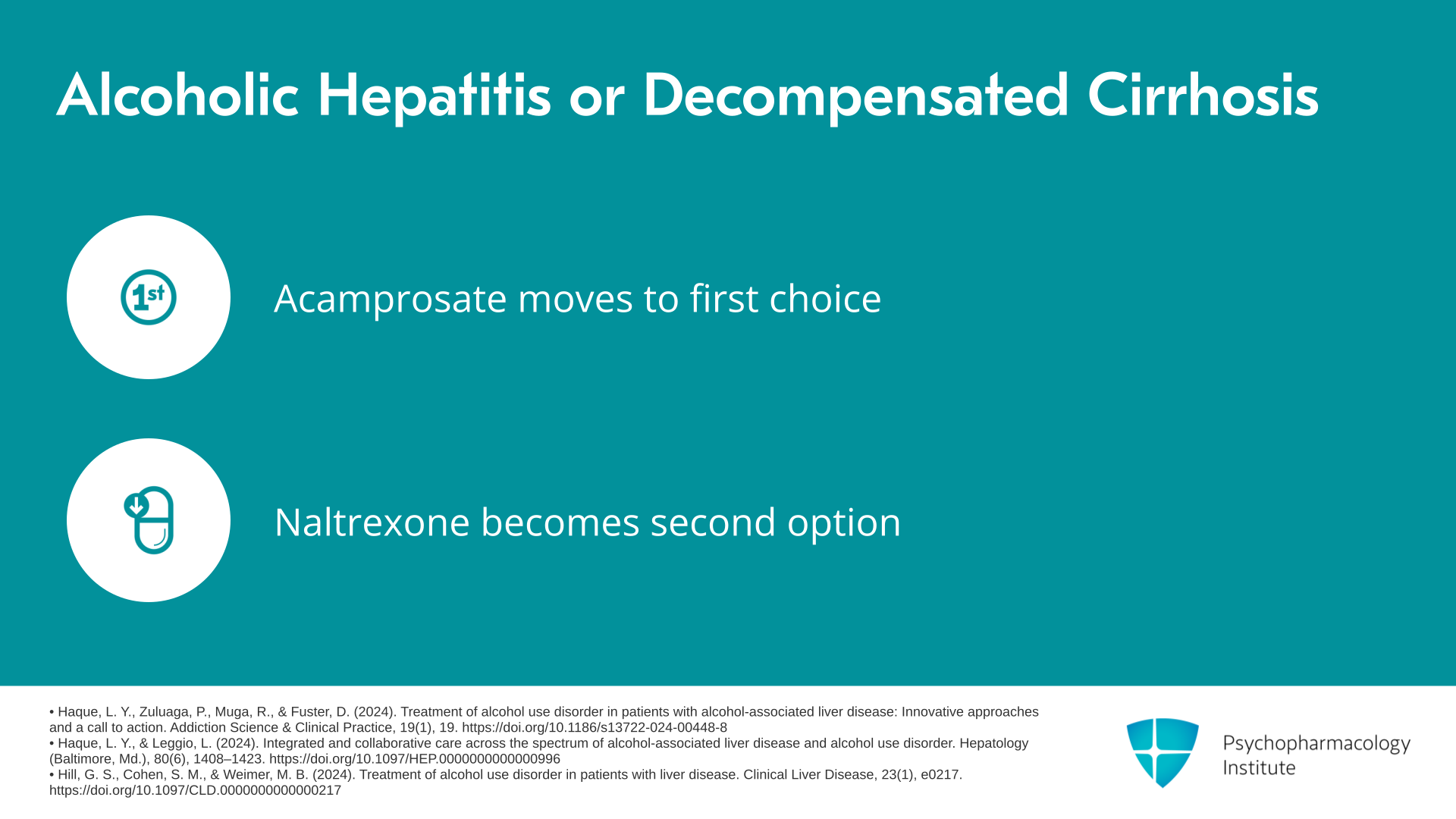
Slide 8 of 14
If there is alcoholic hepatitis or decompensated cirrhosis, so alcoholic hepatitis doesn’t mean cirrhosis, it means you’ve got a severe inflammatory reaction in the liver or decompensated cirrhosis, now you switch. You move acamprosate to the head of the pack, then naltrexone, and finally the non-FDA-approved medications.
References:
- Haque, L. Y., Zuluaga, P., Muga, R., & Fuster, D. (2024). Treatment of alcohol use disorder in patients with alcohol-associated liver disease: Innovative approaches and a call to action. Addiction Science & Clinical Practice, 19(1), 19. https://doi.org/10.1186/s13722-024-00448-8
- Haque, L. Y., & Leggio, L. (2024). Integrated and collaborative care across the spectrum of alcohol-associated liver disease and alcohol use disorder. Hepatology (Baltimore, Md.), 80(6), 1408–1423. https://doi.org/10.1097/HEP.0000000000000996
- Hill, G. S., Cohen, S. M., & Weimer, M. B. (2024). Treatment of alcohol use disorder in patients with liver disease. Clinical Liver Disease, 23(1), e0217. https://doi.org/10.1097/CLD.0000000000000217
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 9 of 14
If there is mild alcohol use disorder, in this algorithm, many would say use brief intervention first with peer support. Consider medications if that’s not effective.
References:
- Haque, L. Y., Zuluaga, P., Muga, R., & Fuster, D. (2024). Treatment of alcohol use disorder in patients with alcohol-associated liver disease: Innovative approaches and a call to action. Addiction Science & Clinical Practice, 19(1), 19. https://doi.org/10.1186/s13722-024-00448-8
- Haque, L. Y., & Leggio, L. (2024). Integrated and collaborative care across the spectrum of alcohol-associated liver disease and alcohol use disorder. Hepatology (Baltimore, Md.), 80(6), 1408–1423. https://doi.org/10.1097/HEP.0000000000000996
- Hill, G. S., Cohen, S. M., & Weimer, M. B. (2024). Treatment of alcohol use disorder in patients with liver disease. Clinical Liver Disease, 23(1), e0217. https://doi.org/10.1097/CLD.0000000000000217
Slide 10 of 14
So that I think is a good guide. There are some things for the non-FDA-approved medications. If topiramate is not tolerated or if gabapentin has not been effective or if baclofen is not tolerated, then you adjust the use of which of those you use.
References:
- Haque, L. Y., Zuluaga, P., Muga, R., & Fuster, D. (2024). Treatment of alcohol use disorder in patients with alcohol-associated liver disease: Innovative approaches and a call to action. Addiction Science & Clinical Practice, 19(1), 19. https://doi.org/10.1186/s13722-024-00448-8
- Haque, L. Y., & Leggio, L. (2024). Integrated and collaborative care across the spectrum of alcohol-associated liver disease and alcohol use disorder. Hepatology (Baltimore, Md.), 80(6), 1408–1423. https://doi.org/10.1097/HEP.0000000000000996
- Hill, G. S., Cohen, S. M., & Weimer, M. B. (2024). Treatment of alcohol use disorder in patients with liver disease. Clinical Liver Disease, 23(1), e0217. https://doi.org/10.1097/CLD.0000000000000217
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
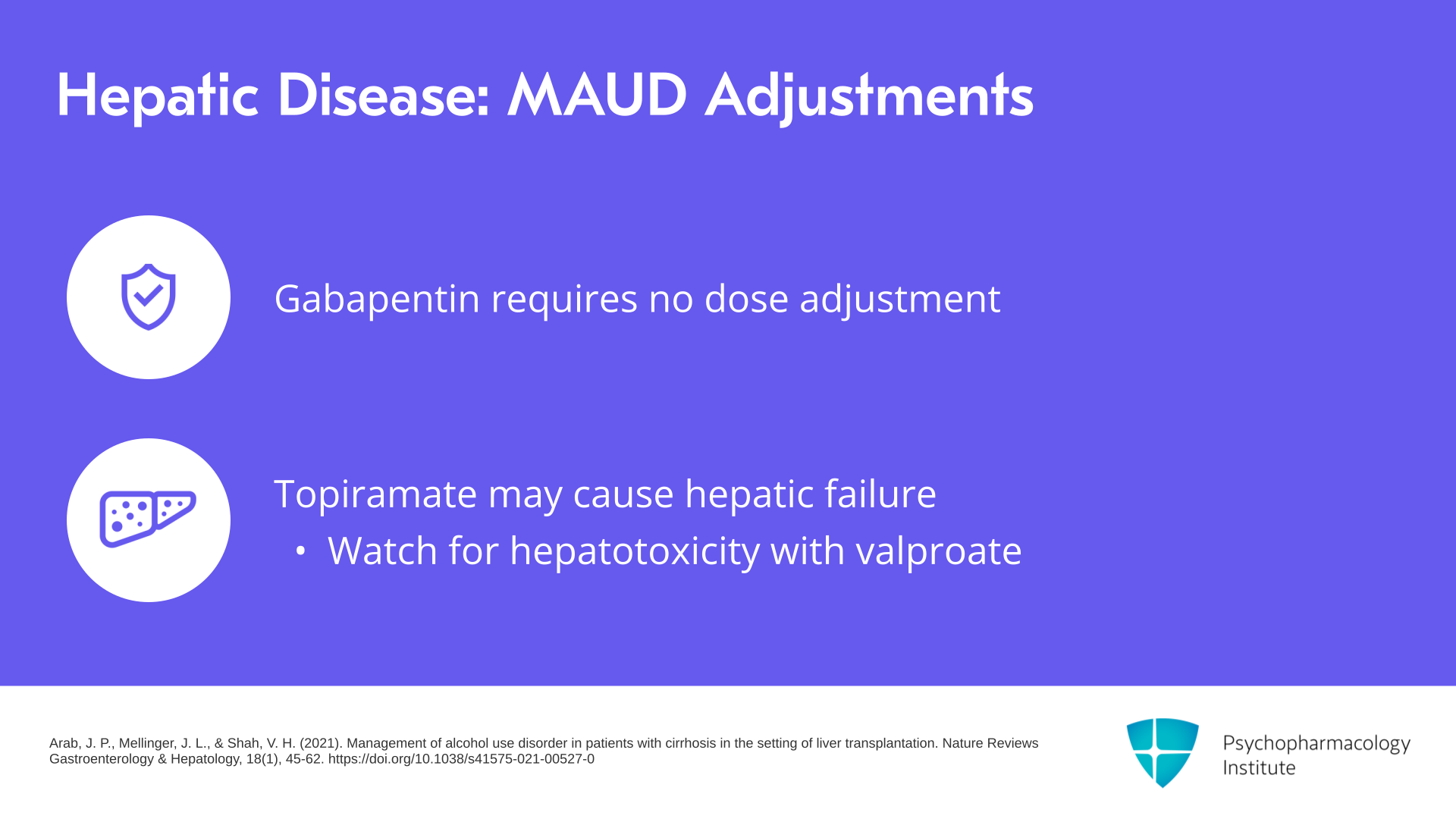
Slide 11 of 14
For hepatic disease with the MAUD adjustments, if there’s impaired liver function, this isn’t just transaminases going up but impaired liver metabolism, gabapentin, prescribing information doesn’t indicate any hepatotoxicity or the need for dose adjustment. For topiramate, there’s very rare hepatic failure. Those have included fatalities. Since it’s metabolized by the CYP450 enzyme 3A4 especially if used with valproate, watch for hepatotoxicity.
References:
- Arab, J. P., Mellinger, J. L., & Shah, V. H. (2021). Management of alcohol use disorder in patients with cirrhosis in the setting of liver transplantation. Nature Reviews Gastroenterology & Hepatology, 18(1), 45-62. https://doi.org/10.1038/s41575-021-00527-0
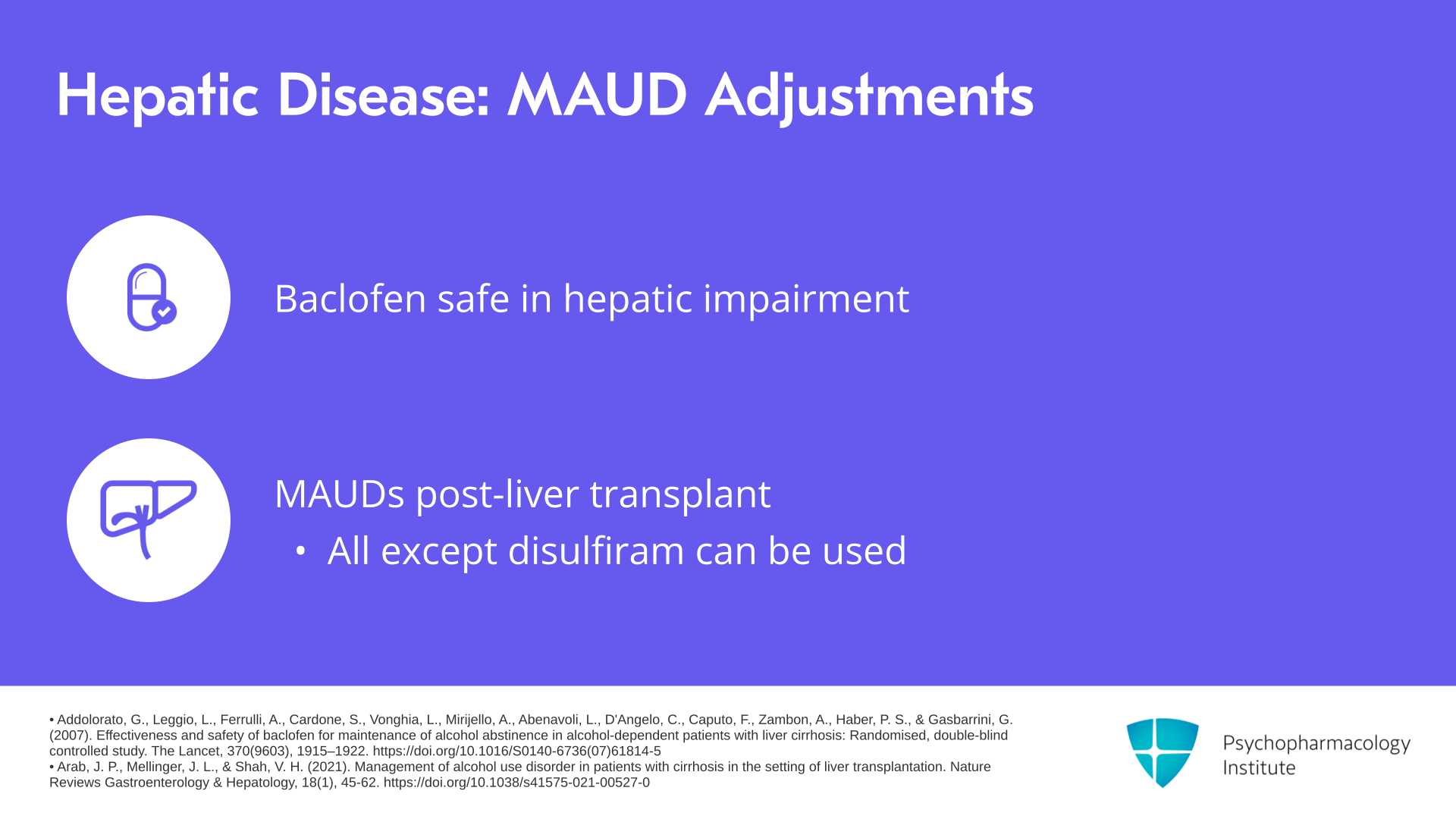
Slide 12 of 14
Baclofen, there are no formal recommendations for dosing adjustments with hepatic impairment because there’s limited hepatic metabolism but there are some case reports of acute hepatitis and clinical trials overall suggest it’s safe in alcoholic liver disease including advanced liver disease. And except for disulfiram, all MAUD agents can be used in liver transplant recipients with the caveats discussed earlier. So once you had a liver transplant and restored liver function, any of the agents except disulfiram could be used. It doesn’t interact with posttransplant immunosuppressants.
References:
- Arab, J. P., Mellinger, J. L., & Shah, V. H. (2021). Management of alcohol use disorder in patients with cirrhosis in the setting of liver transplantation. Nature Reviews Gastroenterology & Hepatology, 18(1), 45-62. https://doi.org/10.1038/s41575-021-00527-0
- Addolorato, G., Leggio, L., Ferrulli, A., Cardone, S., Vonghia, L., Mirijello, A., Abenavoli, L., D'Angelo, C., Caputo, F., Zambon, A., Haber, P. S., & Gasbarrini, G. (2007). Effectiveness and safety of baclofen for maintenance of alcohol abstinence in alcohol-dependent patients with liver cirrhosis: Randomised, double-blind controlled study. The Lancet, 370(9603), 1915–1922. https://doi.org/10.1016/S0140-6736(07)61814-5
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 13 of 14
The key points in this section are: The choice of MAUD should be tailored to individual patient goals. Do they want abstinence or do they want reduced consumption? And this is something the field is moving toward allowing those with alcohol use disorder to consider reduced consumption as their goal. Look at past treatment history and medical comorbidities also to guide your choice.
Slide 14 of 14
If a prior medication for alcohol use disorder has been effective and is well tolerated, restart with that agent. If prior trials of an MAUD have been adequate and they failed, then move on to the next best option. Naltrexone overall is the best first-line option unless there’s decompensated cirrhosis and in which case choose acamprosate.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.