Paroxetine (Paxil, Seroxat, Aropax) is an SSRI approved for major depressive disorder and most anxiety disorders. In clinical practice, many clinicians use it for patients with anxious depression. However, this observation hasn’t been specifically studied in randomized controlled trials. Regarding its side effects profile, important features include higher risk for sexual dysfunction, discontinuation syndrome , and weight gain.
Pharmacodynamics
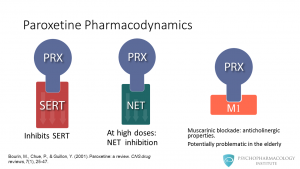
Paroxetine inhibits the reuptake of serotonin by blocking the SERT transporter. The drug also can inhibit the norepinephrine transporter, but this happens at high doses. So far, no clear clinical implications for this noradrenergic feature have been described. The other property paroxetine has is its ability to block muscarinic receptors, this causes the drug to have anticholinergic effects. The importance for this in clinical practice is that central anticholinergic effects can trigger cognitive impairment in the elderly. Watch a related video: The Mechanism of Action of SSRIs
Clinical uses: FDA-approved indications
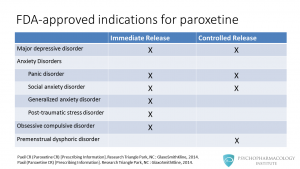
Here we have a list of FDA-approved indications for the two paroxetine formulations, immediate release and controlled release. We’ll explore the differences in more detail in a minute. For now, let’s just focus on the clinical uses. Paroxetine is approved for major depressive disorder and most anxiety disorders. Regarding anxiety disorders, the two formulations are approved for the treatment of panic disorder and social anxiety disorder. Paroxetine immediate release is approved for obsessive compulsive disorder, generalized anxiety disorder and posttraumatic stress disorder. The controlled release formulation is approved for premenstrual dysphoric disorder.
Pharmacokinetics
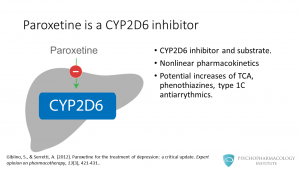
Among the SSRIs, paroxetine is one of the most potent CYP2D6 inhibitors. It is a substrate for and an inhibitor of this isoenzyme. Paroxetine has nonlinear pharmacokinetics, this means that higher doses can produce disproportionately greater plasma drug concentrations as the enzyme becomes saturated. By inhibiting CYP2D6, paroxetine has the potential to increase concentrations of tricyclic antidepressants, phenothiazines and type 1C antiarrythmics.
Adverse effects
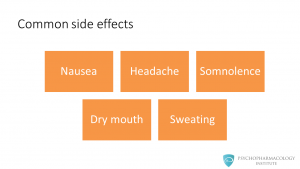
The most common side effects are: • Nausea • Headache • Somnolence • Dry mouth • Sweating Nausea can affect treatment adherence, we’ll see in a minute what the manufacturer did to reduce this side effect.
All SSRIs can cause sexual dysfunction. However, this is more problematic with paroxetine than with other SSRIs. This is a dose-dependent side effect and we should consider it before prescribing paroxetine.
Weight gain also needs to be considered as a possibility when prescribing paroxetine. In a recent review paroxetine was identified as one of the antidepressant with highest risk of producing weight gain.
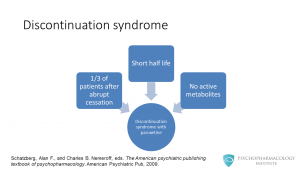
The other potential problem is the possibility of discontinuation syndrome. Although this syndrome can appear with most antidepressants, it can be more problematic with paroxetine. It has been reported that around one-third of patients who stop the drug abruptly can develop discontinuation syndrome. A possible explanation for this is the fact that paroxetine has a short life and no active metabolites.
Prescribing information

The usual dosage range for depression is between 20 to 50 mg/day for the immediate release formulation and between 25-62.5 mg/day for the controlled release formulation.
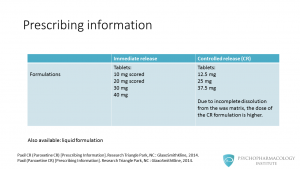
In this table, we can see the most relevant differences between the two formulations. The immediate release formulation is available as tablets: 10 mg scored, 20 mg scored, 30 mg and 40 mg. Controlled release paroxetine is available in tablets of 12.5 mg, 25 mg and 37.5 mg. You might be wondering: why doses are different? The reason is that due to incomplete dissolution of the matrix of the controlled release version, the dose needs to be higher. There is also available a liquid formulation.
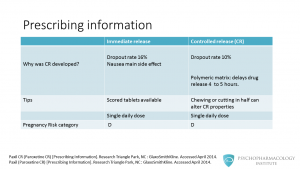
The next question that you might be asking is: why was the CR version developed? One of the most common side effects in clinical trials was nausea, this caused a dropout rate of 16%. So, the manufacturer came up with a polymeric matrix formulation that according to two clinical trials had a lower incidence of nausea. This resulted in a dropout rate of 10 %. This formulation slows the dissolution rate to 4 to 5 hours and delays the release of active paroxetine until the tablets have left the stomach. Another difference is that the immediate release formulation is available as scored tablets. This is important since paroxetine controlled release shouldn’t be chewed or cut in half, as this can alter its properties. Both formulations are dosed as single daily dose. The manufacturer recommends that patients take the medication usually in the morning, but it can be given in the evening too. Lastly, paroxetine is labeled as pregnancy risk category D (this means that there is positive evidence or risk to the human fetus).
Other SSRI videos
- The Psychopharmacology of Fluoxetine: An Illustrated Review for Prescribers
- Fluvoxamine Essentials: Mechanism of Action, Indications, Pharmacokinetics and Dosing
- Sertraline Essentials: Mechanism of Action, Indications, Pharmacokinetics and Dosing
- Citalopram and Escitalopram: A Summary of Key Differences and Similarities
Note: this article can also be found in Spanish “
Paroxetina: farmacodinamia, indicaciones, efectos adversos, farmacocinética y posología
“
References
- Bourin, M., Chue, P., & Guillon, Y. (2001). Paroxetine: a review . CNS drug reviews, 7(1), 25-47.
- Paxil CR (Paroxetine CR) [Prescribing Information]. Research Triangle Park, NC : GlaxoSmithKline.
- Paxil (Paroxetine CR) [Prescribing Information]. Research Triangle Park, NC : GlaxoSmithKline.
- Gibiino, S., & Serretti, A. (2012). Paroxetine for the treatment of depression: a critical update . Expert opinion on pharmacotherapy, 13(3), 421-431.
- Dent, Robert, et al. “ Changes in body weight and psychotropic drugs: a systematic synthesis of the literature .” PloS one 7.6 (2012): e36889.
- Schatzberg, Alan F., and Charles B. Nemeroff, eds. The American psychiatric publishing textbook of psychopharmacology . American Psychiatric Pub, 2009.



