Introduction: Basic Concepts
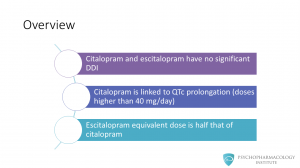
Let me highlight some useful points before getting into more detail about these two medications. • Citalopram and escitalopram have no significant drug-drug interactions. This is because of their low potential to inhibit CYP 450 isoenzymes. • Citalopram is linked to QT prolongation. This drug was initially approved to be used in a range from 20 to 60 mg/day. In 2011, the FDA recommended against its use in doses higher than 40 mg/day. • Regarding prescribing tips, escitalopram equivalent dose is half that of citalopram
Pharmacology
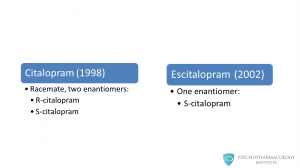
Citalopram was approved in 1998 for the treatment of depression. It is produced as a racemate, this means that it is a mixture of two stereoisomers: R-citalopram and S-citalopram. Escitalopram is only one enantiomer, s-citalopram. This drug was approved in 2002 and was developed with the goal of better tolerability.
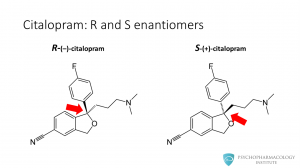
This slide shows the two enantiomers of citalopram. The red arrow points at the drug’s chiral center and its two possible isomers: R-citalopram and S-citalopram.
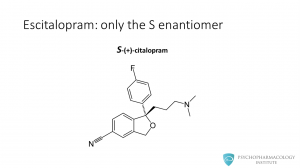
Escitalopram is the S enantiomer, without the R enantiomer. –
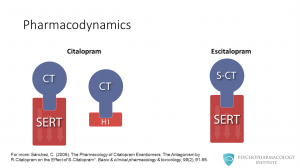
Both drugs are among the most selective of the SSRI class. They don’t have significant affinity for muscarinic, dopaminergic or norepinephrine receptors. In addition to SERT inhibition, citalopram is a mild antagonist at histamine 1 receptors. Escitalopram is also a SERT inhibitor, but doesn’t block histamine 1 receptors.
Indications and Off-Label Uses
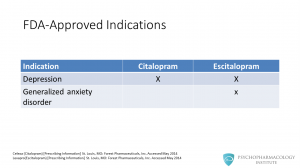
Regarding FDA approved indications, both SSRIs are approved for depression. Citalopram was approved in 1998 and escitalopram in 2002. Escitalopram is also approved for generalized anxiety disorder.

Other off-label uses include:
- OCD
- Panic disorder
- PTSD
- Social anxiety disorder
- Premenstrual dysphoric disorder
Pharmacokinetics
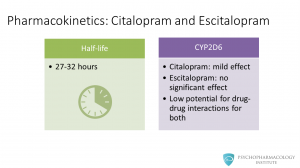
The pharmacokinetics of citalopram and escitalopram is very similar. Both have a half-life of around one day: 27 to 32 hours. This is a feature shared with most SSRIs, there are two exceptions to this: fluvoxamine and fluoxetine. Regarding CYP 450 isoenzymes, citalopram has a mild effect on CYP2D6, while escitalopram has no significant effect. The potential for drug-drug interactions is low for both drugs.
Adverse Effects

The most commonly reported side effects in clinical trials are nausea and vomiting, increased sweating, dry mouth and headache. This is similar for citalopram and escitalopram. Both drugs are pregnancy category C.
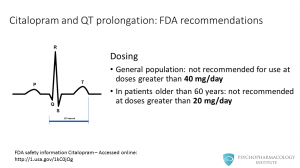
Citalopram is associated with dose-dependent QT interval prolongation. Initially, citalopram was approved to be dosed up to 60 mg/day, but in 2011 the FDA stated that it is not recommended for use at doses greater than 40 mg/day. In 2012, the FDA said that in patients older than 60 years, citalopram is not recommended at doses greater than 20 mg/day. –
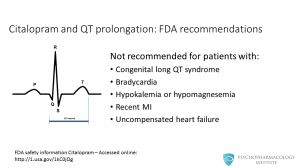
Citalopram is not recommended for patients with: • Congenital long QT syndrome • Bradycardia • Hypokalemia or hypomagnesemia • Recent MI • Uncompensated heart failure

The QTc-prolonging effect with therapeutically equivalent doses of escitalopram was approximately half of that of citalopram, and did not warrant a change in dosing recommendations.
Prescribing Information: The Basics
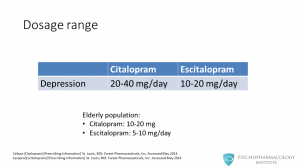
The dosage range for depression for citalopram is 20-40 mg/day, this is according to the new FDA recommendations. Previously, the dosage was 20-60 mg/day. For escitalopram the range is 10-20 mg day. When treating elderly patients, the dose of citalopram is reduced to a range of 10-20 mg/day. For escitalopram, the dose should be from 5 to 10 mg/day.
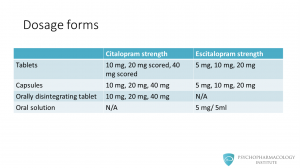
Regarding dosage forms, both drugs are available as tablets and capsules. Citalopram is available as 10 mg, 20 mg scored and 40 mg scored tablets. Escitalopram as 5 mg, 10 mg and 20 mg tablets. Capsules: citalopram capsules of 10, 20 and 40 mg, escitalopram 5, 10 and 20 mg. Citalopram is available as orally disintegrating tablets of 10, 20 and 40 mg. This formulation is not available for escitalopram. There is an escitalopram oral solution of 5 mg/ 5 ml.
Other SSRI videos
- The Psychopharmacology of Fluoxetine: An Illustrated Review for Prescribers
- Fluvoxamine Essentials: Mechanism of Action, Indications, Pharmacokinetics and Dosing
- Sertraline Essentials: Mechanism of Action, Indications, Pharmacokinetics and Dosing
- The Psychopharmacology of Paroxetine: An Illustrated Summary for Prescribers
References and Further Reading
- Sánchez, C. (2006). The Pharmacology of Citalopram Enantiomers: The Antagonism by R‐Citalopram on the Effect of S‐Citalopram*. Basic & clinical pharmacology & toxicology, 99(2), 91-95.
- Celexa (Citalopram) [Prescribing Information] St. Louis, MO: Forest Pharmaceuticals, Inc. Accessed May 2014
- Lexapro(Escitalopram) [Prescribing Information] St. Louis, MO: Forest Pharmaceuticals, Inc. Accessed May 2014
- Schatzberg, Alan F., and Charles B. Nemeroff, eds. The American psychiatric publishing textbook of psychopharmacology. American Psychiatric Pub, 2009
- FDA safety information Citalopram – Accessed online: http://1.usa.gov/1kC0jOg



