Slides and Transcript
Slide 2 of 15
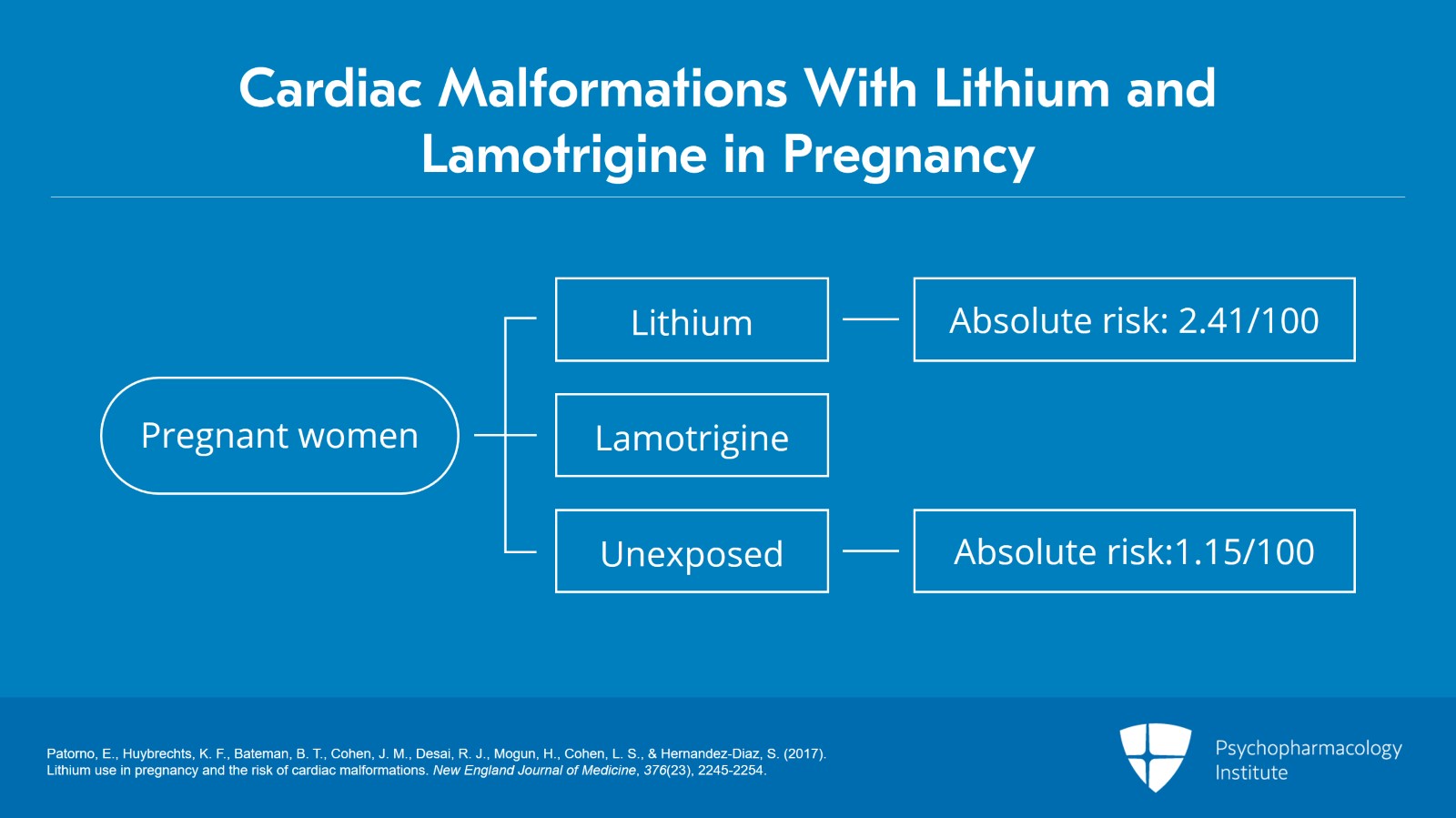
This data comes at least in large part from the New England Journal of Medicine in an article published by Patorno in 2017. What they looked at was overall cardiac malformations and in particular right ventricular outflow defects. This was a large retrospective cohort study which compared infant outcomes in three groups of pregnant women. Group number one were women who are on lithium during pregnancy, group number two were women who are on lamotrigine during pregnancy and group number three were unexposed women to either of these mood stabilizers. And what did they find? When it came to absolute risks, the risk for lithium was 2.41 per 100 exposures versus 1.15 per 100 unexposed women.
References:
- Patorno, E., Huybrechts, K. F., Bateman, B. T., Cohen, J. M., Desai, R. J., Mogun, H., Cohen, L. S., & Hernandez-Diaz, S. (2017). Lithium use in pregnancy and the risk of cardiac malformations. New England Journal of Medicine, 376(23), 2245-2254.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 3 of 15
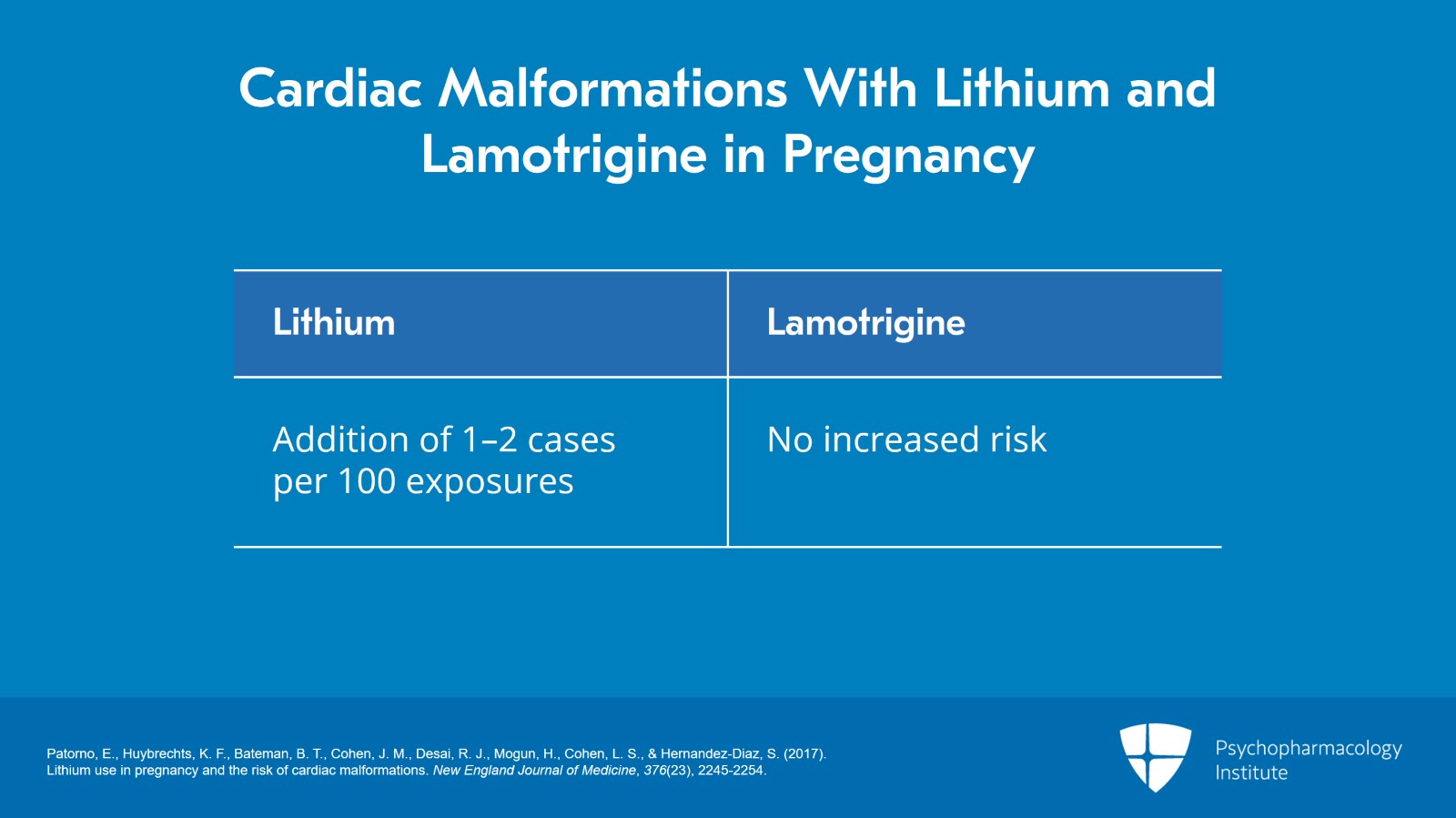
In other words, there was about a one to two addition of cases of overall cardiac malformations per 100 with lithium exposure compared to unexposure. When they looked at lamotrigine, there was no associated increased risk.
References:
- Patorno, E., Huybrechts, K. F., Bateman, B. T., Cohen, J. M., Desai, R. J., Mogun, H., Cohen, L. S., & Hernandez-Diaz, S. (2017). Lithium use in pregnancy and the risk of cardiac malformations. New England Journal of Medicine, 376(23), 2245-2254.
Slide 4 of 15
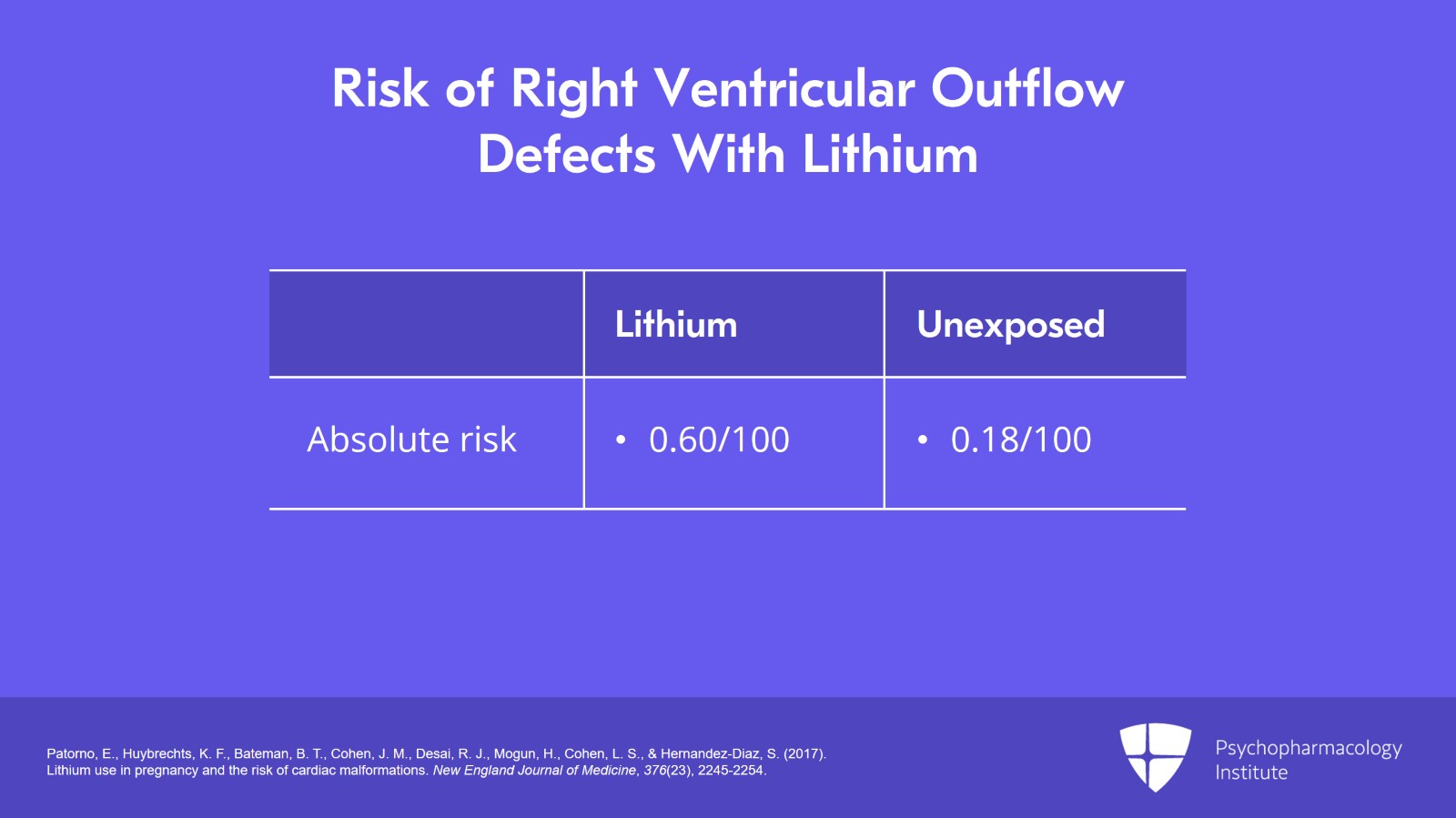
Taking this down a bit and looking at right ventricular outflow defects and we have to remember that Ebstein's anomaly is a secondary outcome of right ventricular outflow defects, the absolute risk was for lithium 0.60 per 100 versus 0.18 per 100 in the unexposed population.
References:
- Patorno, E., Huybrechts, K. F., Bateman, B. T., Cohen, J. M., Desai, R. J., Mogun, H., Cohen, L. S., & Hernandez-Diaz, S. (2017). Lithium use in pregnancy and the risk of cardiac malformations. New England Journal of Medicine, 376(23), 2245-2254.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 5 of 15
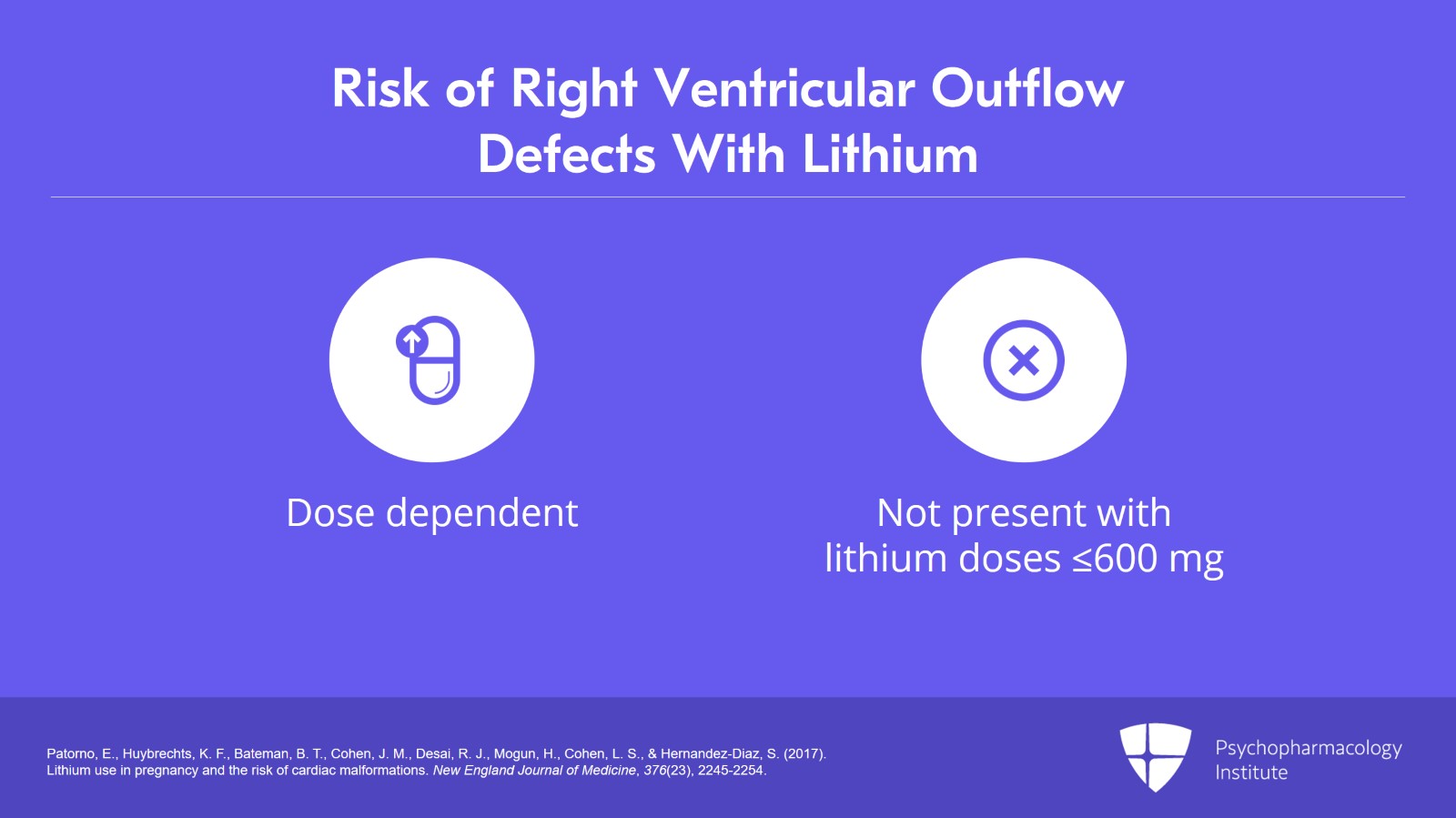
Notice that the risk was dose dependent. There were no right ventricular outflow defects with lithium doses of 600 mg or less. And in fact, in all of the 663 lithium-exposed babies in this study, there was not a single Ebstein's anomaly.
References:
- Patorno, E., Huybrechts, K. F., Bateman, B. T., Cohen, J. M., Desai, R. J., Mogun, H., Cohen, L. S., & Hernandez-Diaz, S. (2017). Lithium use in pregnancy and the risk of cardiac malformations. New England Journal of Medicine, 376(23), 2245-2254.
Slide 6 of 15
Importantly, lithium was not associated with any noncardiac malformations.
References:
- Patorno, E., Huybrechts, K. F., Bateman, B. T., Cohen, J. M., Desai, R. J., Mogun, H., Cohen, L. S., & Hernandez-Diaz, S. (2017). Lithium use in pregnancy and the risk of cardiac malformations. New England Journal of Medicine, 376(23), 2245-2254.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 7 of 15
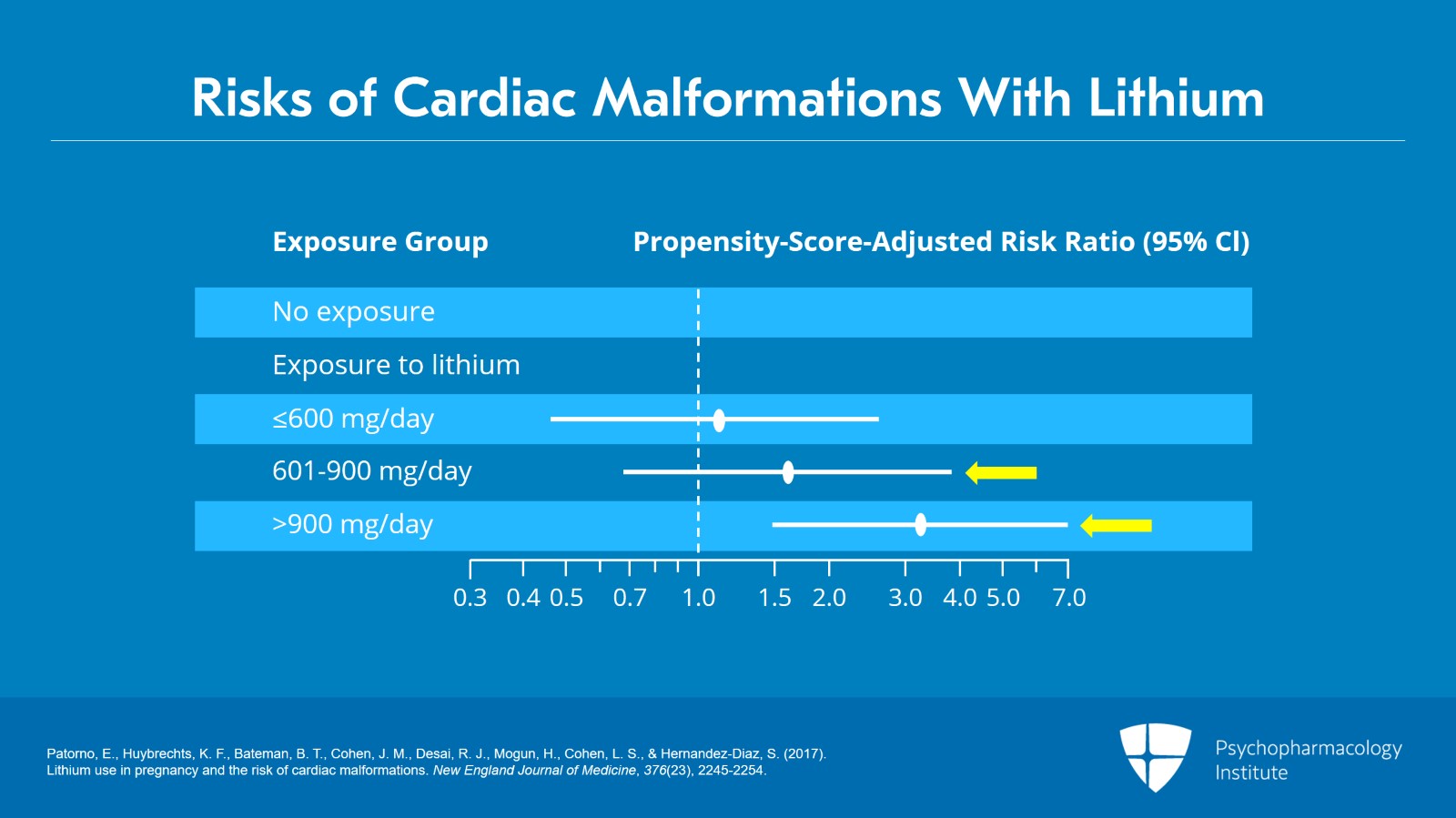
Take a look, if you will, at the next chart which explains lithium absolute and relative risks of cardiac malformations and what you can see is that at greater than 900 mg per day there was a substantially increased risk of cardiac malformations. The risk increased threefold in doses above 900 mg per day. At under 900 mg per day, that risk was much less.
References:
- Patorno, E., Huybrechts, K. F., Bateman, B. T., Cohen, J. M., Desai, R. J., Mogun, H., Cohen, L. S., & Hernandez-Diaz, S. (2017). Lithium use in pregnancy and the risk of cardiac malformations. New England Journal of Medicine, 376(23), 2245-2254.
Slide 8 of 15
So this is, illustrates Patorno's finding that the risk of cardiac malformations with prenatal lithium exposure was dose dependent and became significant at greater than 900 mg per day.
References:
- Patorno, E., Huybrechts, K. F., Bateman, B. T., Cohen, J. M., Desai, R. J., Mogun, H., Cohen, L. S., & Hernandez-Diaz, S. (2017). Lithium use in pregnancy and the risk of cardiac malformations. New England Journal of Medicine, 376(23), 2245-2254.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 9 of 15
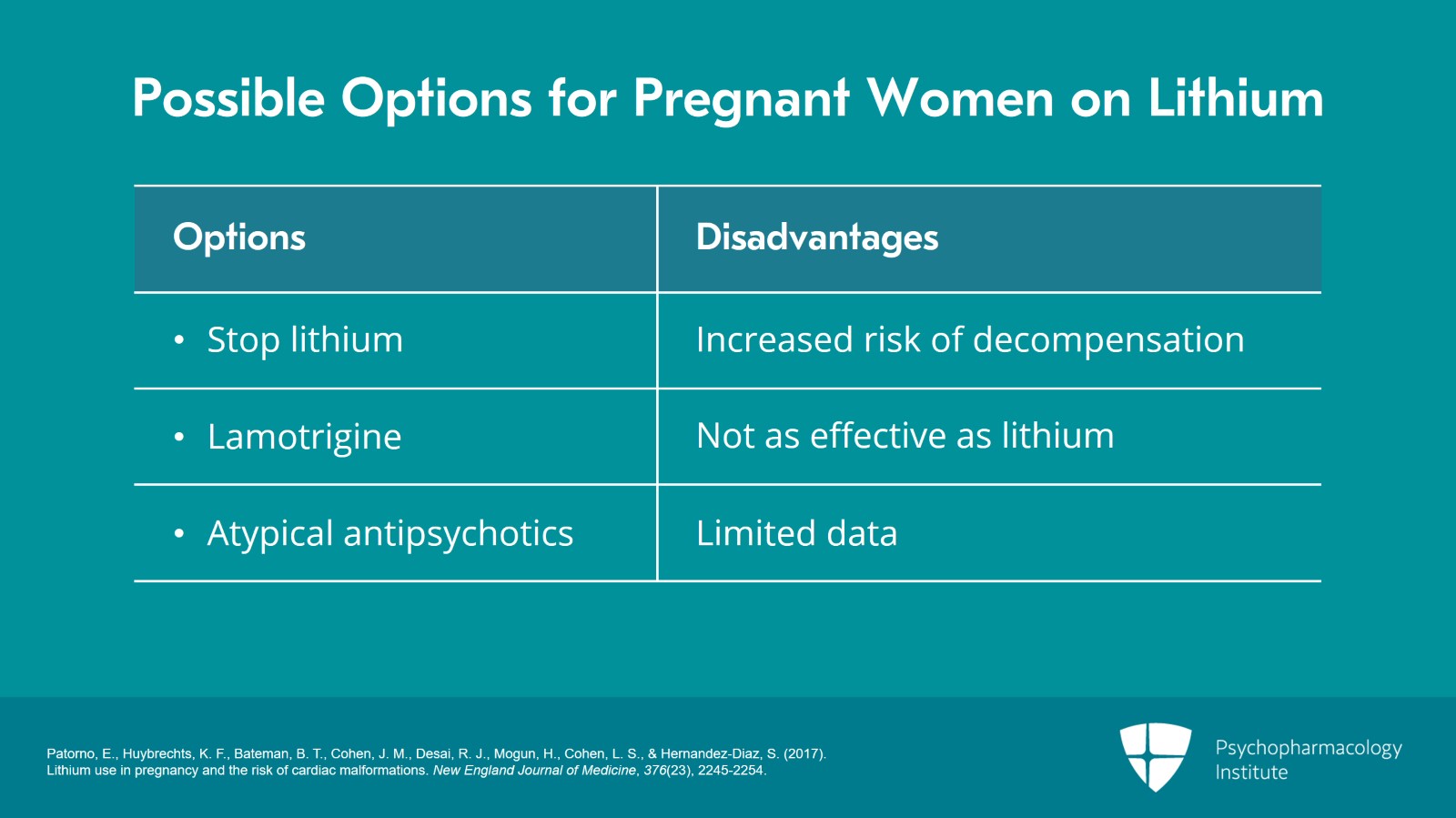
And so some would say you might consider the lowering the dose in the first trimester but let's think about that. What are in fact the options? Well, we could stop lithium but we know that if we do that the risk is dramatically increased for bipolar decompensation particularly in the first trimester but throughout pregnancy. Another option is considering replacing lithium with lamotrigine but remember it's generally not as effective as lithium especially for patients who have mania or mixed mania as part of their history. We could consider an atypical antipsychotic but there's less data for bipolar disorder with atypical antipsychotics. And I will later on talk to you about the risks of both lamotrigine and antipsychotics in pregnancy.
References:
- Patorno, E., Huybrechts, K. F., Bateman, B. T., Cohen, J. M., Desai, R. J., Mogun, H., Cohen, L. S., & Hernandez-Diaz, S. (2017). Lithium use in pregnancy and the risk of cardiac malformations. New England Journal of Medicine, 376(23), 2245-2254.
Slide 10 of 15
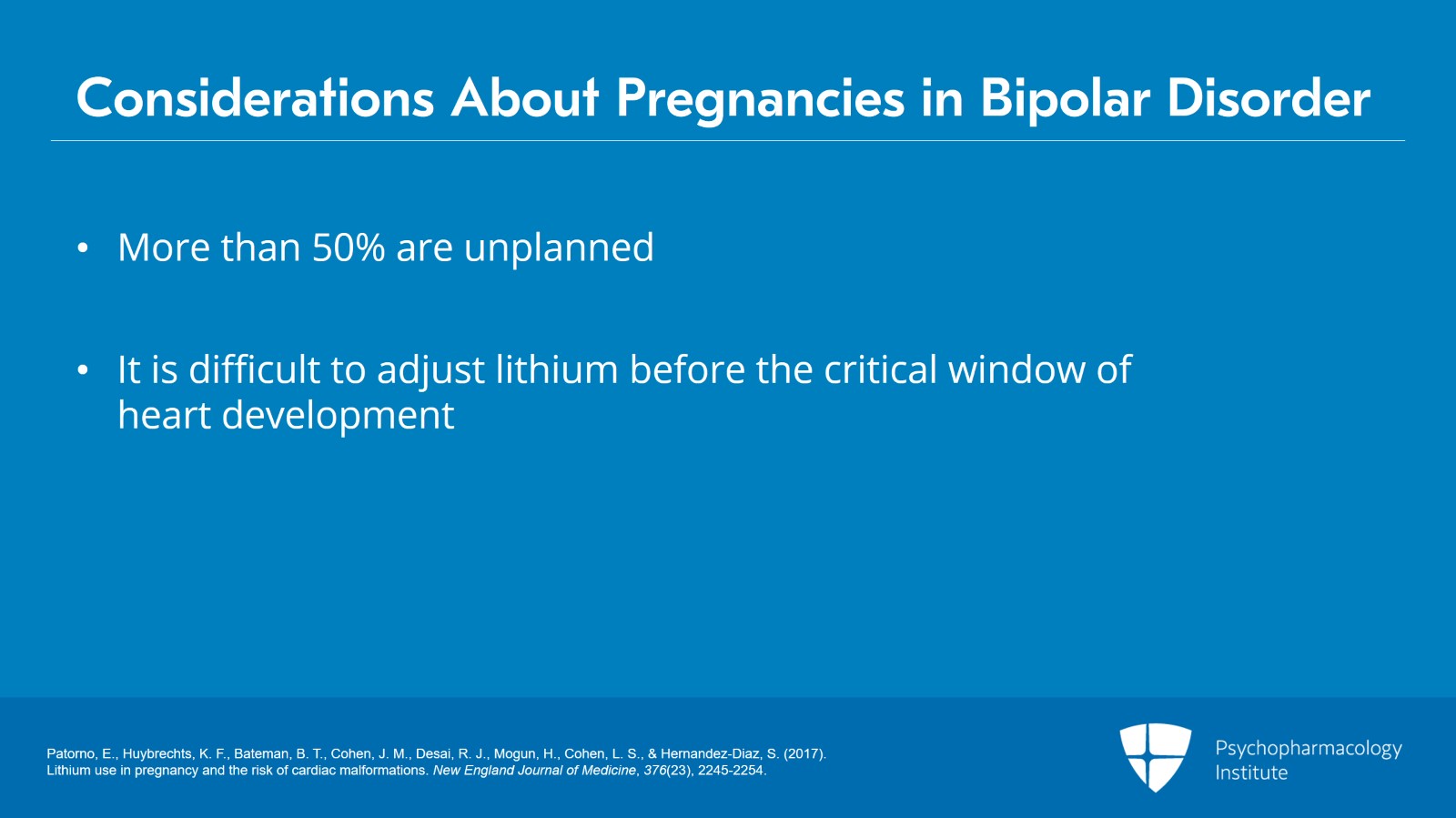
Remember, if you will, that more than 50% of pregnancies are unplanned and it's always difficult to adjust lithium doses after a positive pregnancy test and yet before the critical window of heart development which is four to eight weeks post conception. So we always have to be aware of the early risk for relapse as I've discussed.
References:
- Patorno, E., Huybrechts, K. F., Bateman, B. T., Cohen, J. M., Desai, R. J., Mogun, H., Cohen, L. S., & Hernandez-Diaz, S. (2017). Lithium use in pregnancy and the risk of cardiac malformations. New England Journal of Medicine, 376(23), 2245-2254.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 11 of 15
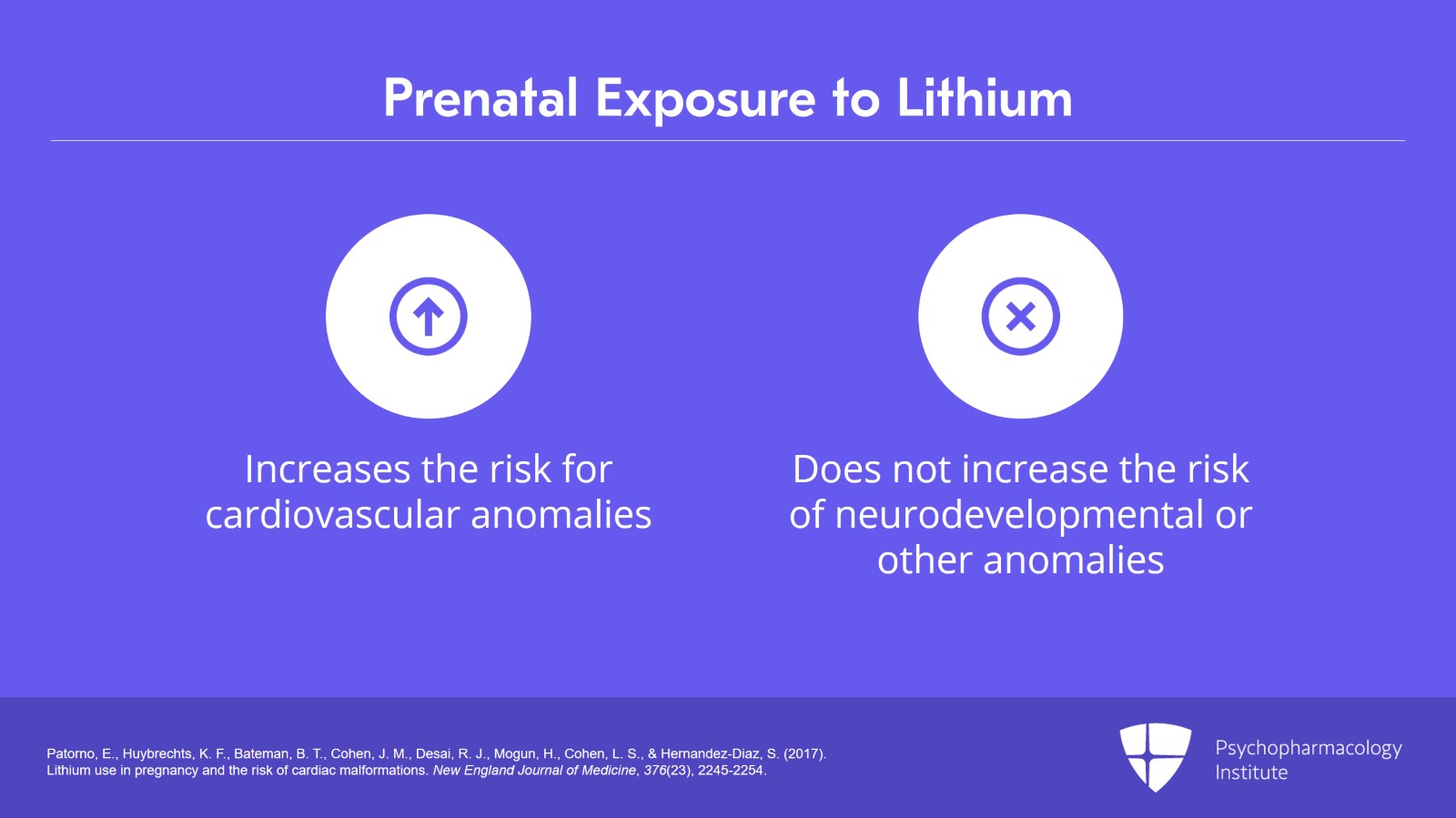
Now, remember prenatal exposure to lithium increases the risk for cardiovascular anomalies does not increase the risk for neurodevelopmental anomalies or other anomalies.
References:
- Patorno, E., Huybrechts, K. F., Bateman, B. T., Cohen, J. M., Desai, R. J., Mogun, H., Cohen, L. S., & Hernandez-Diaz, S. (2017). Lithium use in pregnancy and the risk of cardiac malformations. New England Journal of Medicine, 376(23), 2245-2254.
Slide 12 of 15
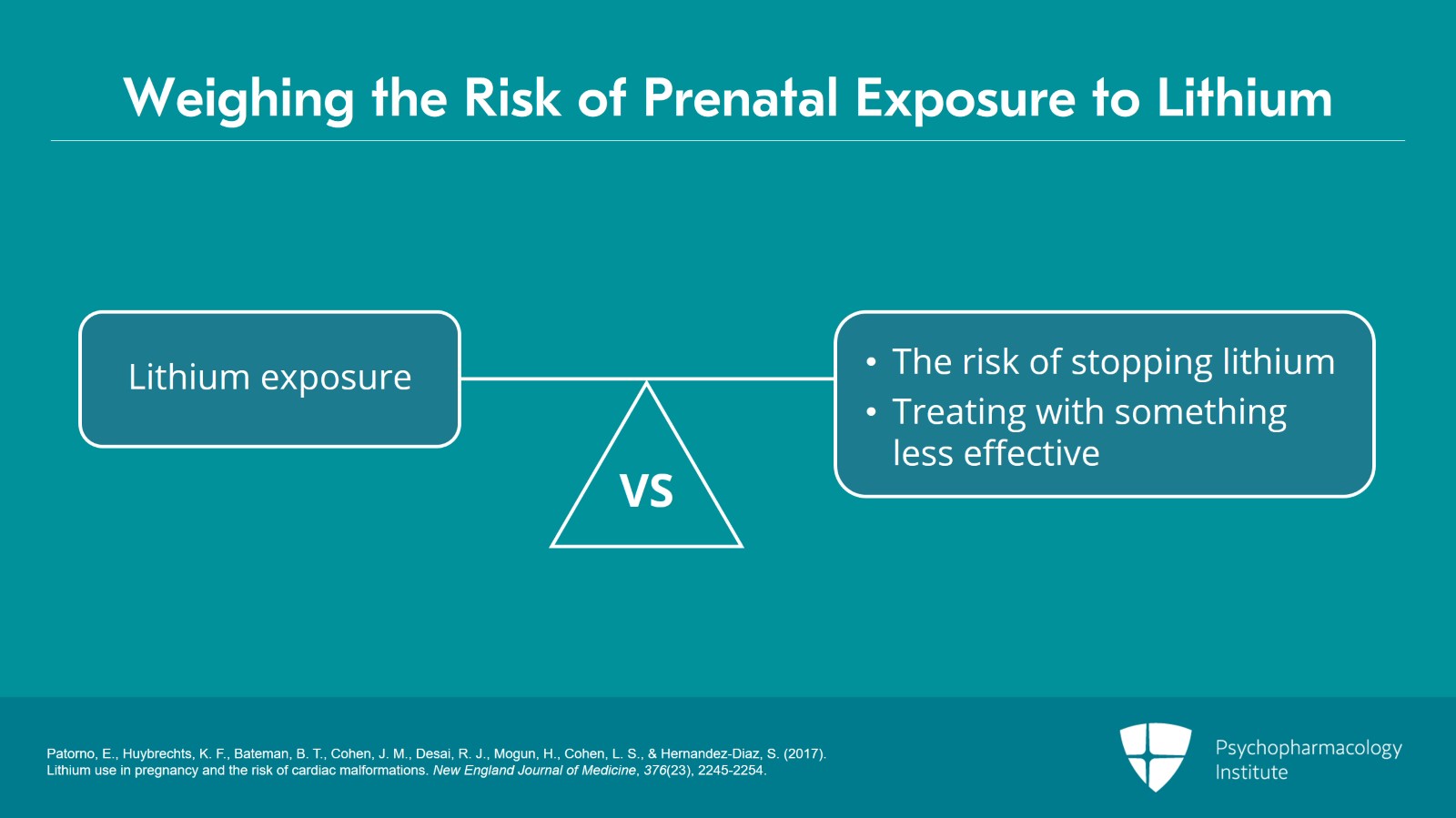
How do we conclude and think about this? You have to weigh the risk of lithium exposure against the risk associated with stopping lithium and not treating the disorder or treating with something that might be less effective or for which we have less data.
References:
- Patorno, E., Huybrechts, K. F., Bateman, B. T., Cohen, J. M., Desai, R. J., Mogun, H., Cohen, L. S., & Hernandez-Diaz, S. (2017). Lithium use in pregnancy and the risk of cardiac malformations. New England Journal of Medicine, 376(23), 2245-2254.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 13 of 15
So what are our key points? Prenatal exposure to lithium increases the risk for cardiovascular anomalies but that risk should be weighed against the risk incurred to the bipolar mother and her offspring by discontinuing lithium.
Slide 14 of 15
Prenatal exposure to lithium does not increase the risk for noncardiac or neurodevelopmental disorders in exposed offspring.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.