Slides and Transcript
Slide 1 of 24
So turning to video 2, naltrexone: Potential indications and administration in children and adolescents.
Slide 2 of 24
Opiate antagonists don’t readily cross the blood-brain barrier but they are potent competitive inhibitors with an affinity for mu-receptors which are found in very high concentration in bronchial smooth muscle and the digestive tract.
So endogenous opioid peptides such as endorphins and enkephalins are present naturally in the brain and are released during times of physical pain or stress.
Opiate antagonists block these effects and this blockade is the mechanism through which their psychiatric effects are derived.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 3 of 24
Opiate antagonists have been observed to reverse hyperphagia and obesity, decrease social aggression, and attenuate drug- or stress-induced stereotypy associated with elevated endogenous opiate levels.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
Slide 4 of 24
Now, in terms of mechanism of action for naltrexone, naltrexone is a potent opioid antagonist with a high affinity for mu-opioid binding sites which completely and reversibly blocks the effects of endogenous and exogenous opiate derivatives.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 5 of 24
And there is more and more evidence regarding the efficacy and side effect profile of naltrexone for psychiatric conditions but again this is mainly derived from adult studies of substance use and there’s considerable variability in response rates.
Indeed, there aren’t any currently FDA-approved pediatric indications for naltrexone.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
Slide 6 of 24
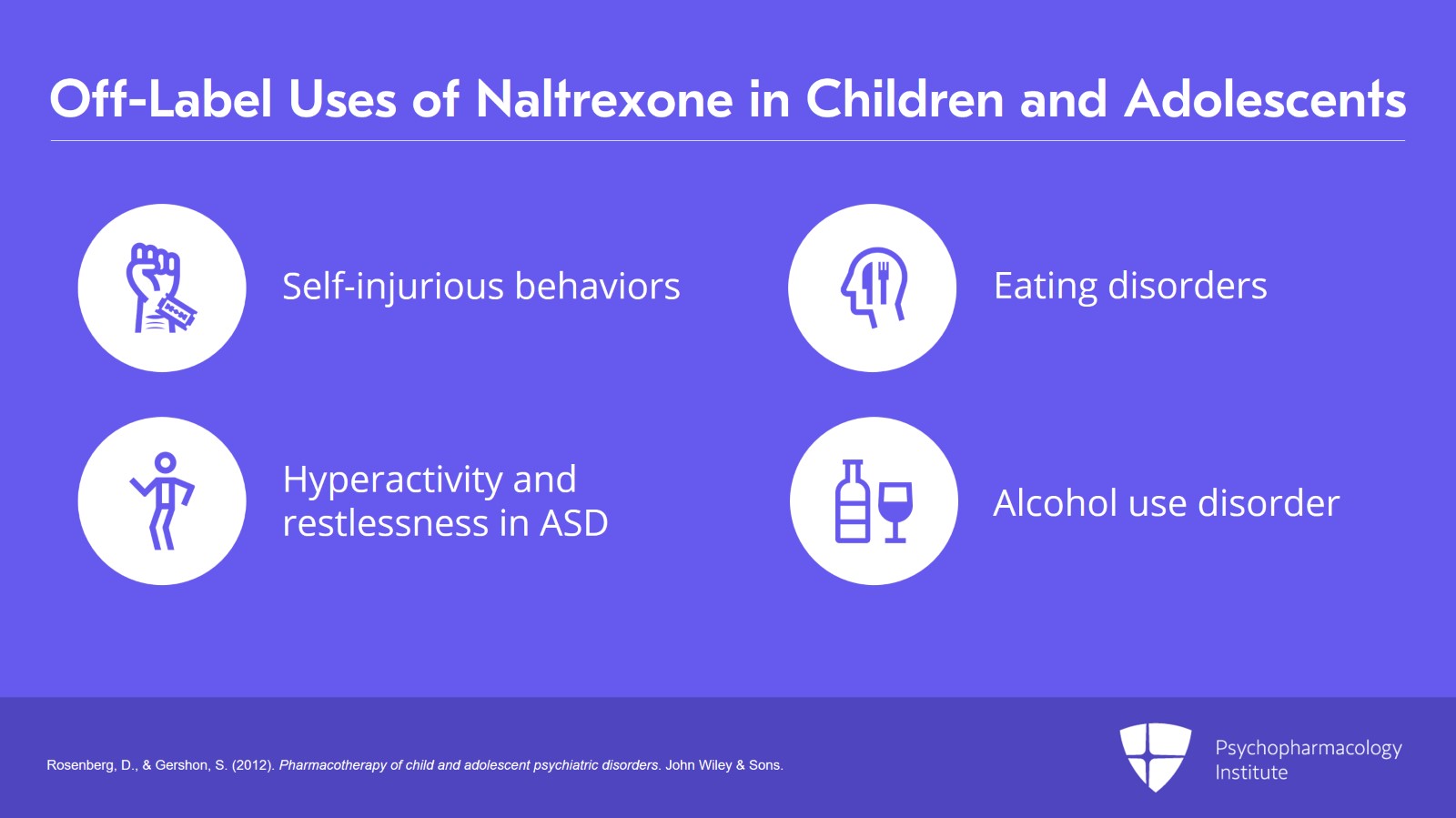
That being said, off-label use regularly occurs in conditions associated with the opioid reward pathway such as self-injurious behaviors, eating disorders, hyperactivity, and restlessness in autism spectrum disorders and alcohol abuse disorders.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 7 of 24
Potential indications, self-injurious behaviors.
This is an area of high attention because elevated levels of endogenous opioids and decreased pain perception are hypothesized to contribute to the etiology or cause of many self-injurious behaviors.
So naltrexone may actually be helpful for reestablishing pain perception and decreasing self-injurious behaviors in individual patients.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
- Sabus, A., Feinstein, J., Romani, P., Goldson, E., & Blackmer, A. (2019). Management of self‐injurious behaviors in children with neurodevelopmental disorders: A pharmacotherapy overview. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 39(6), 645-664.
Slide 8 of 24
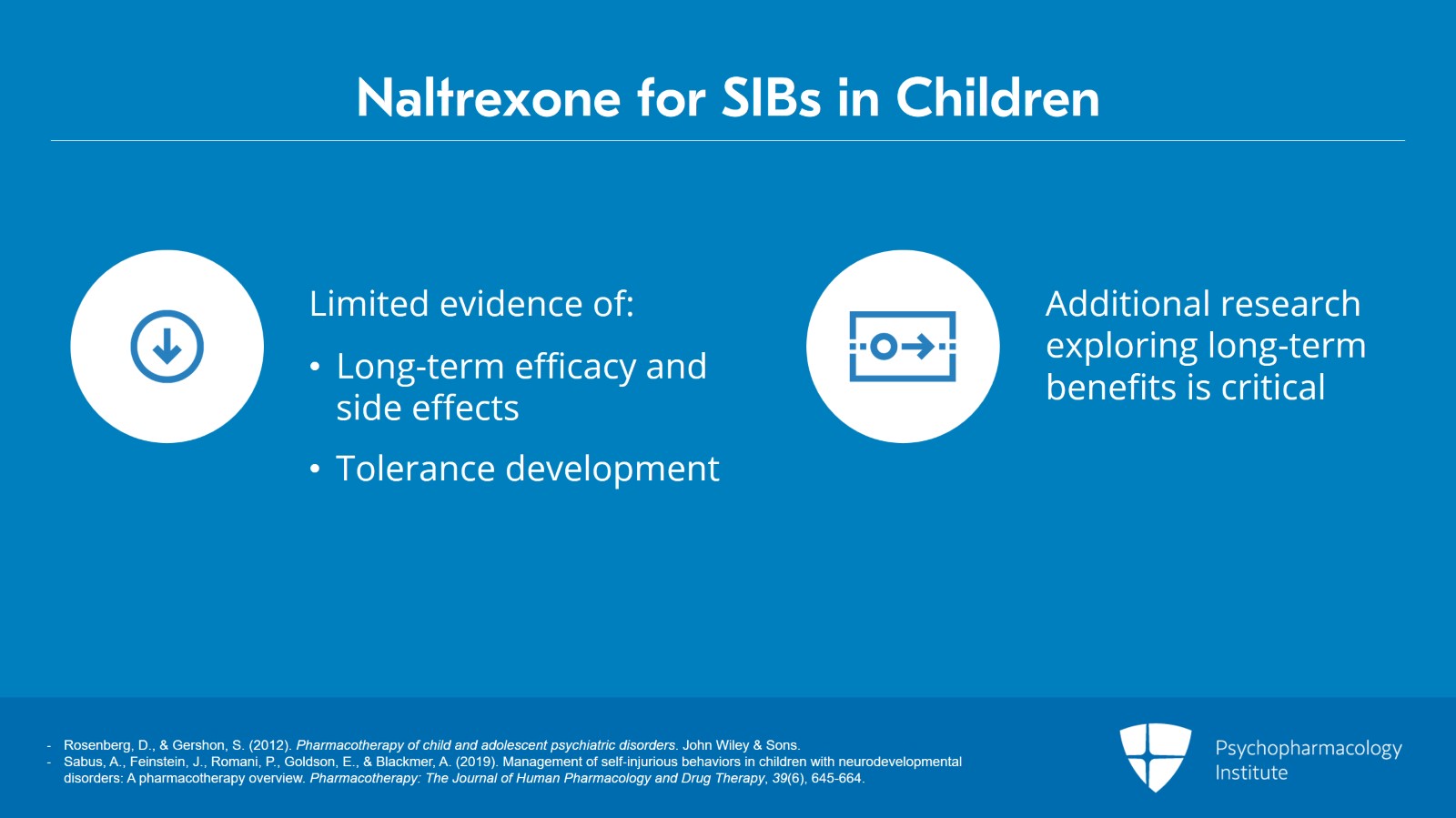
That being said again, there is little evidence of long-term efficacy or information on possible long-term side effects in children. Self-injurious behaviors also persist for many years and it’s unknown whether a tolerance to opiate antagonism can develop.
Several placebo-controlled studies have reported a reduction of self-injurious behaviors with naltrexone treatment but many of these studies have been conducted in very small samples and over a very limited time period and other studies did fail to demonstrate any effect whatsoever.
Thus, additional research exploring long-term clinical benefits is critical.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
- Sabus, A., Feinstein, J., Romani, P., Goldson, E., & Blackmer, A. (2019). Management of self‐injurious behaviors in children with neurodevelopmental disorders: A pharmacotherapy overview. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 39(6), 645-664.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 9 of 24
The current evidence supporting the use of naltrexone for self-injurious behaviors is limited and conflicting. Therefore, naltrexone should be reserved only for extreme cases of self-injurious behavior refractory to other more usual treatment options.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
- Sabus, A., Feinstein, J., Romani, P., Goldson, E., & Blackmer, A. (2019). Management of self‐injurious behaviors in children with neurodevelopmental disorders: A pharmacotherapy overview. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy, 39(6), 645-664.
Slide 10 of 24
Now, naltrexone also has a potential indication for autism spectrum disorder.
And symptoms seen in opioid intoxication such as social withdrawal, stereotypies, and sensory sensitivity led to the hypothesis that there could be abnormalities of the endogenous opioid system playing an etiologic role in autism. As such, naltrexone was considered as a potential treatment for autism.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
- Roy, A., Roy, M., Deb, S., Unwin, G., & Roy, A. (2014). Are opioid antagonists effective in attenuating the core symptoms of autism spectrum conditions in children: A systematic review. Journal of Intellectual Disability Research, 59(4), 293-306.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 11 of 24
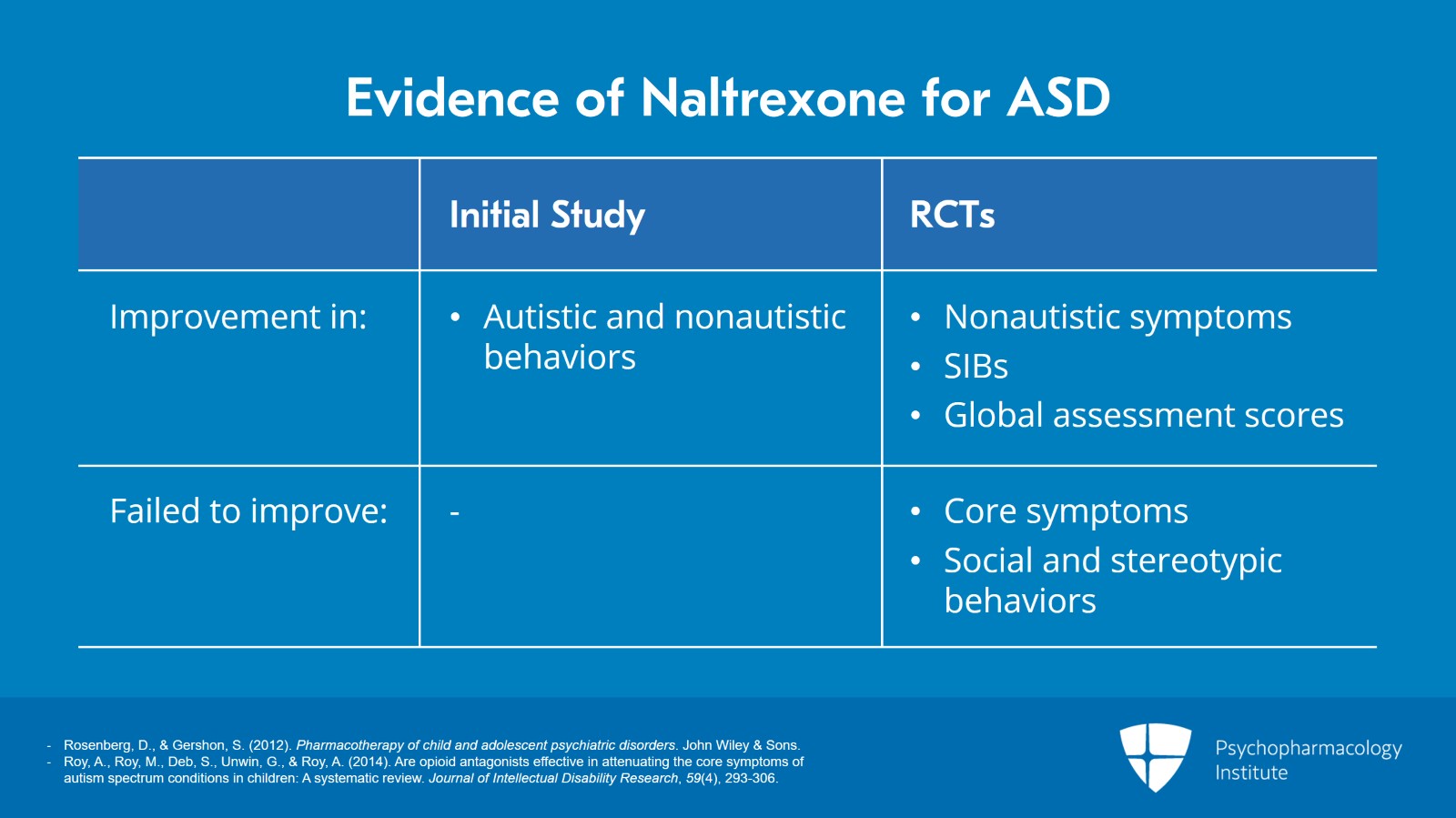
An initial study of naltrexone in autistic children actually found improvements in both autistic, decreased social withdrawal, increased verbal production, reduced stereotype behaviors, and nonautistic that is decreases in restlessness and tantrum symptoms.
Subsequent randomized controlled trials also found that naltrexone produce significant improvement in nonautistic symptoms, impulsivity, hyperactivity, and irritability and led to decreases in self-injurious behaviors and improved global assessment scores.
However, in these same studies, naltrexone failed to improve the core symptoms of autism and had no significant effect on social and stereotypic behaviors.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
- Roy, A., Roy, M., Deb, S., Unwin, G., & Roy, A. (2014). Are opioid antagonists effective in attenuating the core symptoms of autism spectrum conditions in children: A systematic review. Journal of Intellectual Disability Research, 59(4), 293-306.
Slide 12 of 24
And due to significant heterogeneity of available trials, small sample sizes, evidence to support the use of naltrexone for treating the core symptoms of autism is still lacking.
That being said, I find this a very promising area so please stay tuned.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
- Roy, A., Roy, M., Deb, S., Unwin, G., & Roy, A. (2014). Are opioid antagonists effective in attenuating the core symptoms of autism spectrum conditions in children: A systematic review. Journal of Intellectual Disability Research, 59(4), 293-306.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 13 of 24
Now, naltrexone, potential indications for eating disorders. This is also an increasing area of use and study.
Abnormal endorphin levels may underlie the etiology of binge or disordered eating. Naltrexone has been used to treat binge eating and purging in adults. That being said, again, there are a few controlled studies that have been conducted and none have been conducted with adolescent participants.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
- Stancil, S. L., Adelman, W., Dietz, A., & Abdel-Rahman, S. (2019). Naltrexone reduces binge eating and purging in adolescents in an eating disorder program. Journal of Child and Adolescent Psychopharmacology, 29(9), 721-724.
Slide 14 of 24
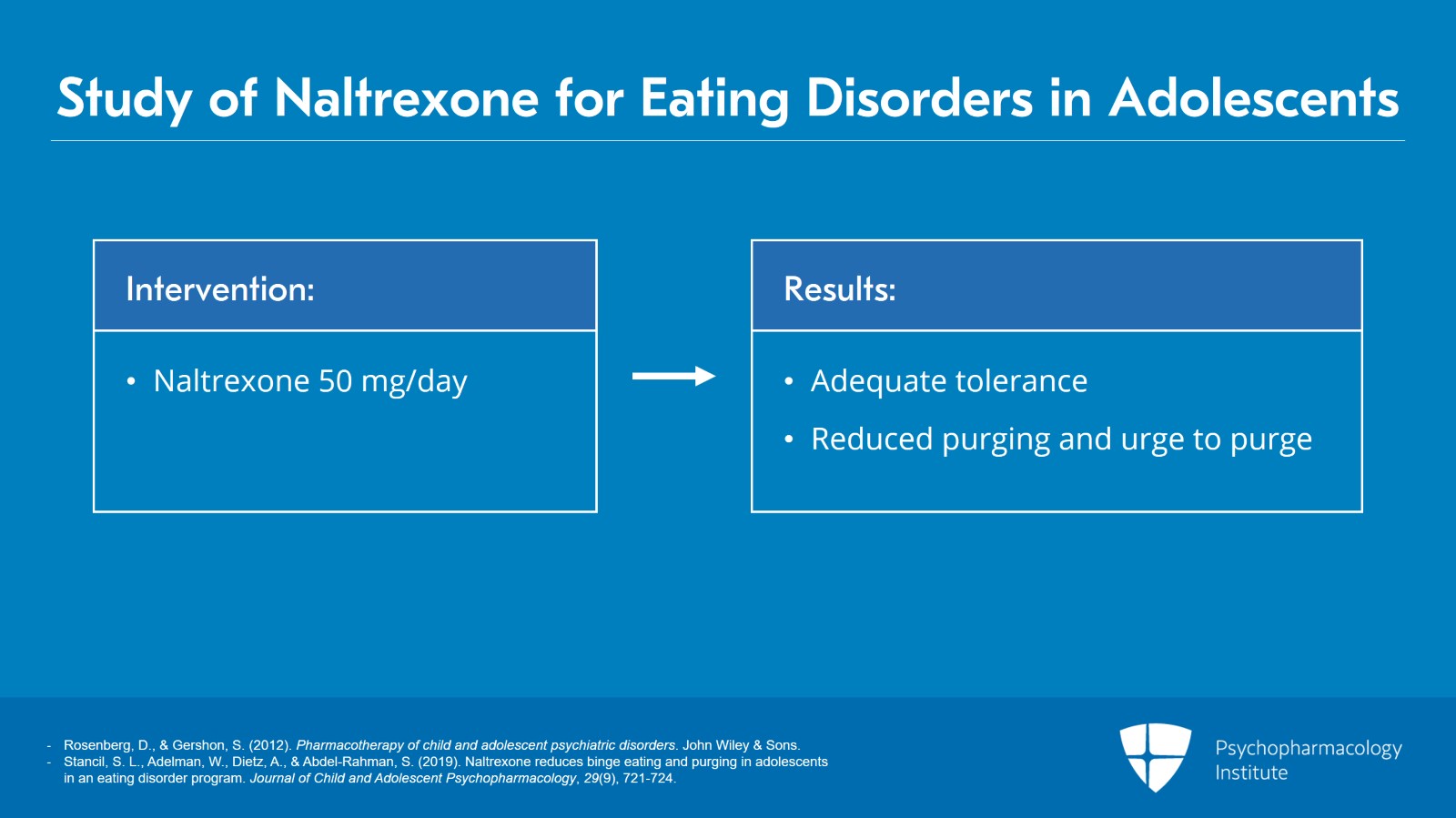
In a recent retrospective chart review of adolescent patients receiving low dose, that means 50 mg per day of naltrexone, it was well tolerated and was associated with reduced purging or urge to purge.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
- Stancil, S. L., Adelman, W., Dietz, A., & Abdel-Rahman, S. (2019). Naltrexone reduces binge eating and purging in adolescents in an eating disorder program. Journal of Child and Adolescent Psychopharmacology, 29(9), 721-724.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 15 of 24
While additional studies are clearly needed, low-dose naltrexone appears to be well tolerated and to reduce symptoms and urges of binge eating and purging with no serious side effects.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
- Stancil, S. L., Adelman, W., Dietz, A., & Abdel-Rahman, S. (2019). Naltrexone reduces binge eating and purging in adolescents in an eating disorder program. Journal of Child and Adolescent Psychopharmacology, 29(9), 721-724.
Slide 16 of 24
Potential indications for alcohol abuse disorder.
The use of naltrexone for alcohol dependence is well established among adult patients. Data on safety and effectiveness with adolescents though are quite limited.
There are results of a small open-label trial suggesting that naltrexone is well tolerated in adolescents seeking treatment and appears to support efficacy in adolescents.
That being said, additional research is needed before naltrexone can be recommended in pediatric populations routinely.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 17 of 24
It is being used but the recommendation at present is to try other more standard interventions prior to naltrexone and when it is used to use a smaller or lower dose.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
Slide 18 of 24
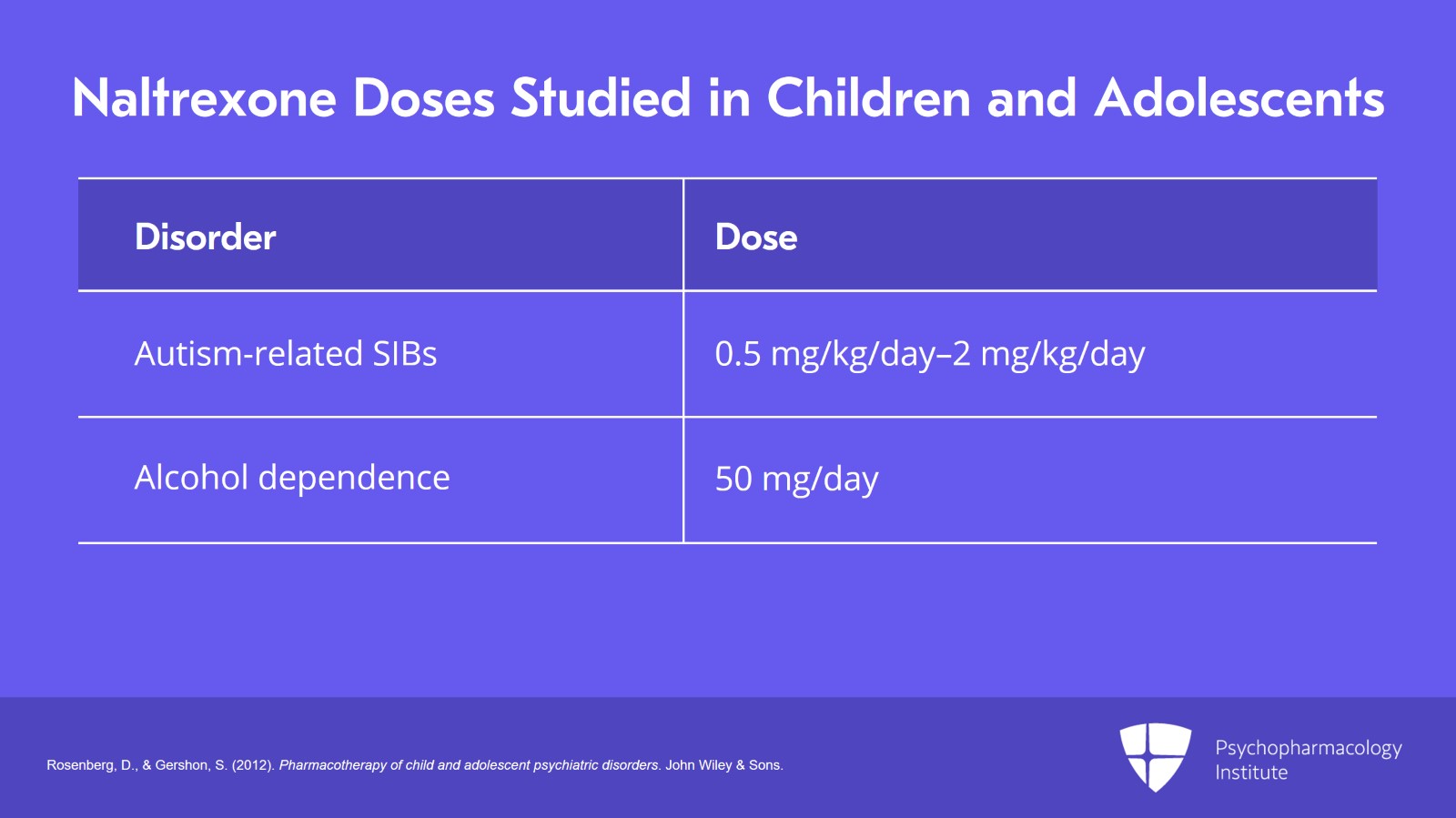
For autism and autism-related self-injurious behaviors, naltrexone has been studied in children in a dosage range from 0.5 to 2 mg/kg/day.
And for alcohol dependence in adolescents, the studies recommend a daily dose of 50 mg per day.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 19 of 24
Side effects.
Mild sedation was the most commonly reported side effect in children as well as insomnia, fatigue, and nausea.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
Slide 20 of 24
Mild hepatic toxicity has also been reported at high doses but not at low doses which still appear to effectively block opioid receptors.
Liver function tests should be considered though before initiating treatment and periodically during chronic treatment to monitor for this rare but obviously concerning effect.
Abuse and dependence of naltrexone has not been described and there are no reported cases of overdose.
References:
- Rosenberg, D., & Gershon, S. (2012). Pharmacotherapy of child and adolescent psychiatric disorders. John Wiley & Sons.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 21 of 24
So key take-home points here are there are currently no FDA-approved pediatric indications for naltrexone.
However, off-label use regularly occurs in conditions associated with abnormalities of the endogenous opioid system such as self-injurious behaviors, eating disorders, autism spectrum disorders, and alcohol use disorders.
Slide 22 of 24
Current evidence supporting the use of naltrexone in children and adolescents is limited and conflicting but low-dose naltrexone appears to effectively block opioid receptors and is well tolerated with few side effects.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.
Slide 23 of 24
Mild hepatic toxicity has been reported at high doses and liver function tests should be considered before initiation of treatment and periodically during chronic treatment.
Free Files
Download PDF and other files
Success!
Check your inbox, we sent you all the materials there.