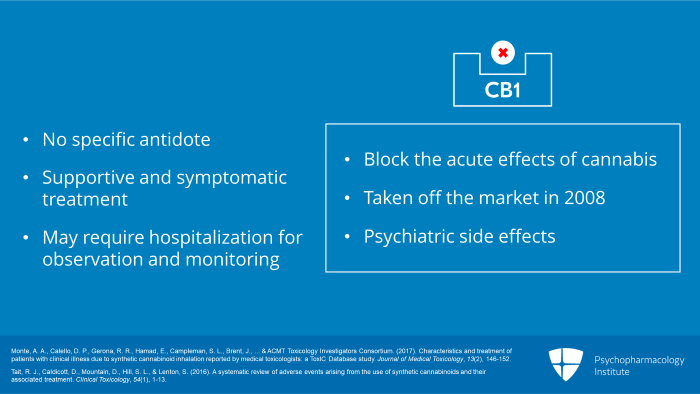
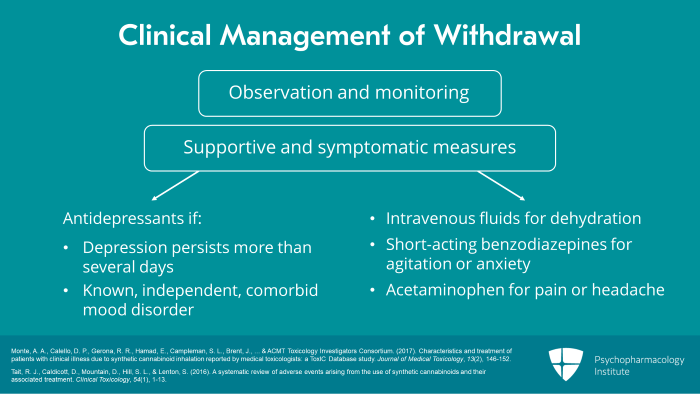
Now, let us move to section four, Synthetic Cannabinoids: Management of Intoxication and Withdrawal. There is no specific antidote for synthetic cannabinoid intoxication. Antagonists of the CB1 receptor which block the acute effects of cannabis were available in the past but were taken off the market in 2008 because of psychiatric side effects. Thus, current clinical management is solely supportive and symptomatic. Because of the possibly long duration of action, patients may require hospitalization for observation and monitoring until mental status, physiological parameters and laboratory tests return to normal.
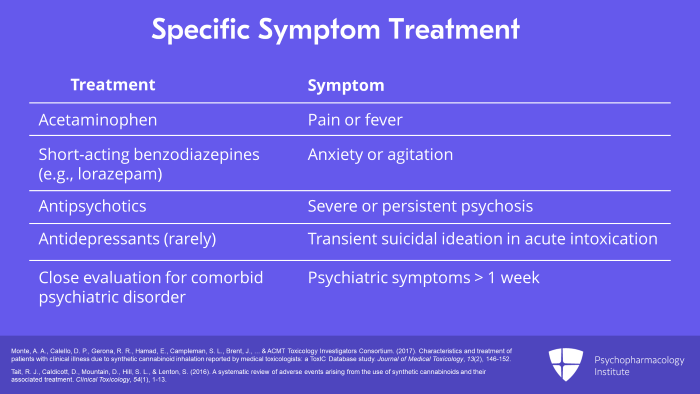
Specific symptoms are treated as clinically indicated, for example, acetaminophen for pain or fever, a short-acting benzodiazepine such as lorazepam for anxiety or agitation and then an antipsychotic for severe or persistent psychosis. Transient suicidal ideation may occur during the acute intoxicated state but rarely requires antidepressant treatment. Psychiatric symptoms persisting longer than one week warrant close evaluation for a comorbid psychiatric disorder. There are currently no consensus clinical practice guidelines for the treatment of synthetic cannabinoid intoxication. So the clinician must rely on his or her own clinical judgment with specialty consultation as appropriate.
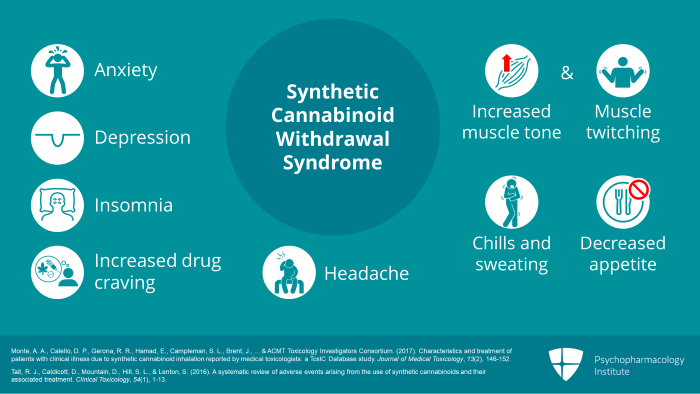
Abrupt cessation of chronic or excessive synthetic cannabinoid use often results in a withdrawal syndrome with features similar to but more intense than those associated with cessation of plant cannabis use. Typical symptoms include anxiety, depression, insomnia, increased drug craving, increased muscle tone or muscle twitching, chills and sweating, decreased appetite and headache. These are also the typical signs and symptoms of plant cannabis withdrawal.
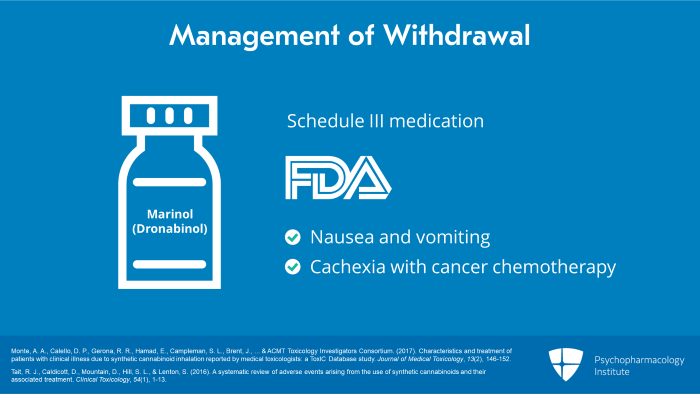
Withdrawal from plant cannabis has been treated successfully in clinical trials with synthetic THC, known generically as dronabinol, which is legally available in the US as Marinol. This is a schedule 3 oral medication which is FDA approved for the treatment of nausea and vomiting or cachexia associated with cancer
chemotherapy.

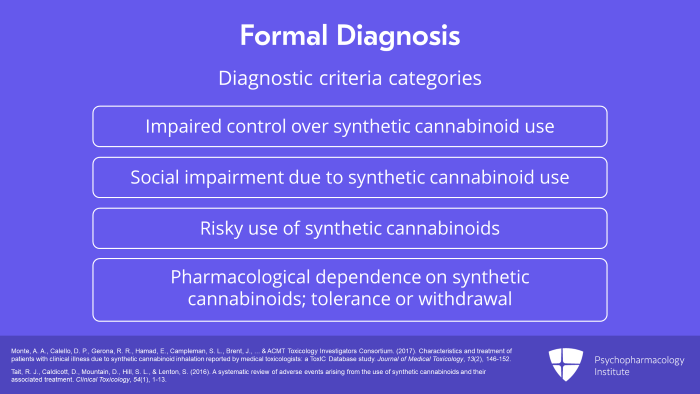
There is no such formal diagnosis in DSM-5 or ICD-10 but a diagnosis could be made by generalizing from the criteria provided for cannabis or other substance use disorder. These diagnostic criteria fall into four broad categories: First, impaired control over synthetic cannabinoid use. Second, social impairment due to synthetic cannabinoid use. Third, risky use of synthetic cannabinoids. And finally, pharmacological dependence on synthetic cannabinoids as evidenced by tolerance or withdrawal.
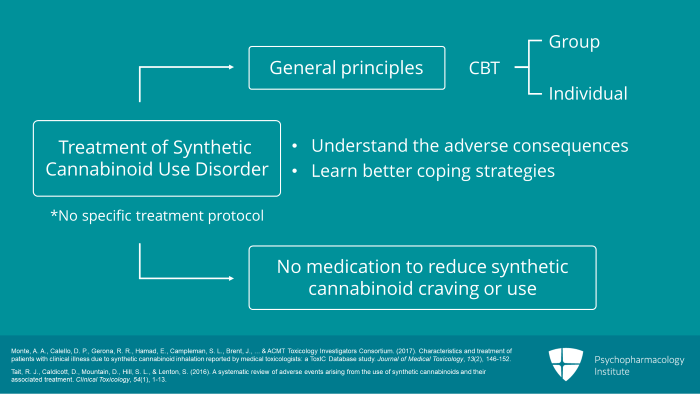
There are no specific treatment protocols for synthetic cannabinoid use disorder. Treatment is based on general principles of treatment for any substance use disorder. This usually involves cognitive behavioral therapy, either in a group or individual setting, focused on getting the patient to understand the adverse consequences of their substance use and to learn better coping strategies for dealing with drug craving and situations which promote substance use. No medication has been shown in clinical trials to reduce craving for or use of synthetic cannabinoids.

To summarize, the key points, first, intoxication and withdrawal syndromes often resemble those from plant cannabis but may be more intense and longer lasting. Treatment of intoxication and withdrawal is supportive and symptomatic as no specific antidotes are available.
References:
- Monte, A. A., Calello, D. P., Gerona, R. R., Hamad, E., Campleman, S. L., Brent, J., … & ACMT Toxicology Investigators Consortium. (2017). Characteristics and treatment of patients with clinical illness due to synthetic cannabinoid inhalation reported by medical toxicologists: a ToxIC Database study. Journal of Medical Toxicology, 13(2), 146-152.
- Tait, R. J., Caldicott, D., Mountain, D., Hill, S. L., & Lenton, S. (2016). A systematic review of adverse events arising from the use of synthetic cannabinoids and their associated treatment. Clinical Toxicology, 54(1), 1-13.



